- Title
-
The Cdc14B phosphatase contributes to ciliogenesis in zebrafish
- Authors
- Clément, A., Solnica-Krezel, L., and Gould, K.L.
- Source
- Full text @ Development
|
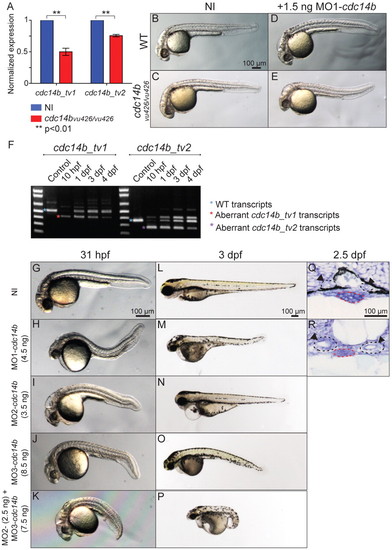
Morphological phenotype resulting from Cdc14B loss of function. (A) Quantitative RT-PCR comparing levels of cdc14b_tv1 and cdc14b_tv2 expression in wild-type and cdc14bvu426 homozygous mutant zebrafish embryos at 10 hpf. Data are mean ± s.e.m. Statistics were carried out using Student′s t-test. (B-E) Wild-type (B,D) and cdc14bvu426 homozygous mutant (C,E) non-injected (NI) control embryos (B,C) and injected with 1.5 ng of MO1-cdc14b (D,E). (F) RT-PCR showing the efficiency of 4.5 ng MO1-cdc14b. (G-P) Non-injected control and cdc14b MO-injected embryos/larvae at 31 hpf (G-K) and 3 dpf (L-P). (Q,R) Sections (5 μm) through the kidney of non-injected control (Q) and MO1-cdc14b-injected (R) larvae at 2.5 dpf. The medial tubules (arrowheads) and the gut are outlined in black and red, respectively. PHENOTYPE:
|
|
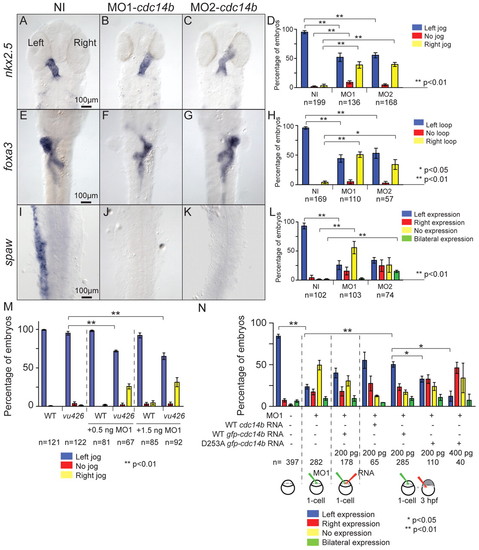
Effect of inhibition of Cdc14B function on LR asymmetry. (A-L) In situ hybridization and expression pattern quantification in non-injected and cdc14b MO-injected zebrafish embryos of nkx2.5 (A-D), foxa3 (E-H) and spaw (I-L) at 31 hpf, 50 hpf and 19 hpf, respectively. (M) Quantification of LR asymmetry defects in non-injected and 0.5 ng or 1.5 ng MO1-cdc14b-injected wild-type and cdc14bvu426 homozygous mutant embryos at 31 hpf using cmlc2:GFP transgene. (N) Rescue of the LR asymmetry phenotype in MO1-cdc14b-injected embryos. LR asymmetry defects were quantified using spaw expression pattern at 18 hpf. Numbers of embryos analyzed are indicated (n). Data are mean ± s.e.m. Statistics were carried out using Student′s t-test (D,H,L,M) and paired Student′s t-test (N), and are only presented for the left-sided heart class in M and the spaw left expression class in N. EXPRESSION / LABELING:
PHENOTYPE:
|
|
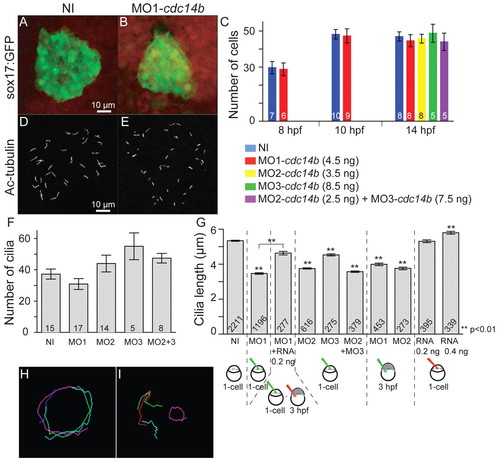
Cdc14B loss- and gain-of-function effect on the KV. (A,B) KV at 10 hpf visualized using Sox17-GFP. (C) The number of cells in the KV was determined in non-injected and cdc14b MO-injected zebrafish embryos at the indicated times. (D,E) Cilia in the KV at 14 hpf visualized with an anti-acetylated-tubulin (Ac-tubulin) antibody. (F,G) Cilia numbers (F) and cilia length (G) were determined at 14 hpf. The numbers of embryos (C,F) and cilia (G) analyzed are indicated. Data are mean ± s.e.m. Statistics were carried out using Student′s t-test. Unless otherwise shown, statistics were calculated against the NI sample. (H,I) Particle paths in the KV of a single non-injected (H) or MO1-cdc14b-injected (I) embryo at 14 hpf. Three embryos were analyzed per condition. EXPRESSION / LABELING:
PHENOTYPE:
|
|
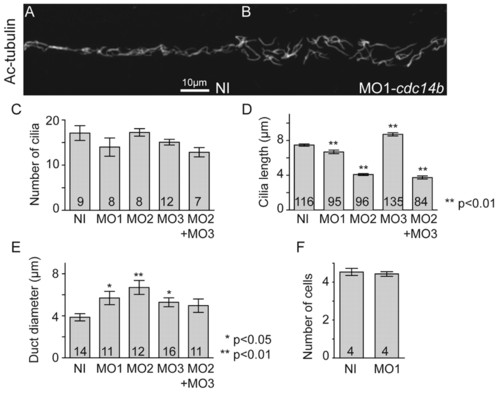
Cdc14B loss-of-function effect on kidney cilia. (A,B) Cilia in the kidney duct at 27 hpf visualized with an anti-Ac-tubulin antibody. (C,D) Cilia numbers (C) and length (D) were determined at 27 hpf. (E) Kidney duct diameter was measured at 27 hpf. (F) Number of cells in sections of the kidney tubules at 2.5 dpf. The numbers of embryos (C,E,F) and cilia (D) analyzed are indicated in each column. Data are mean ± s.e.m. Statistics were carried out using Student′s t-test. PHENOTYPE:
|
|
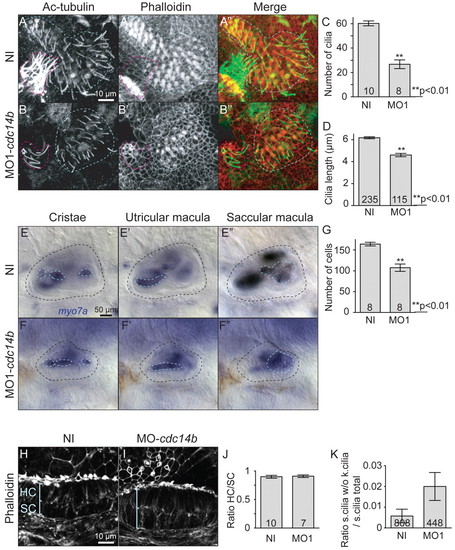
Cdc14B loss-of-function effect on inner ear kinocilia. (A-B′) Saccular macula at 4 dpf visualized with phalloidin and an anti-Ac-tubulin antibody. The latter structure is outlined in blue; the lateral crista is outlined in red. (C,D) Cilia numbers (C) and length (D) were determined at 4 dpf. (E-F′) myo7a in situ hybridization in the inner ear at 4 dpf. (G) The number of cells in the saccular macula was determined in non-injected and MO1-cdc14b-injected larvae at 4 dpf. (H,I) Structure of the utricular macula in non-injected (H) and MO1-cdc14b-injected (I) larvae at 4 dpf visualized by phalloidin. (J) Ratio between the numbers of hair cells (HC) and supporting cells (SC) at 4 dpf. (K) Ratio between the numbers of stereocilia (s.cilia) not associated to kinocilia (k.cilia) and the total number of stereocilia. The numbers of larvae (C,G,J), cilia (D) and hair cells (K) analyzed are indicated. Data are mean ± s.e.m. Statistics were carried out using Student′s t-test. |
|
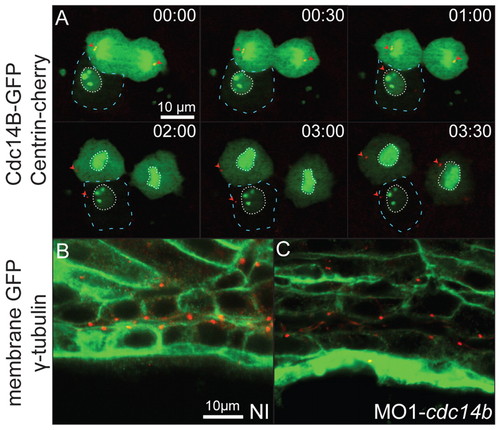
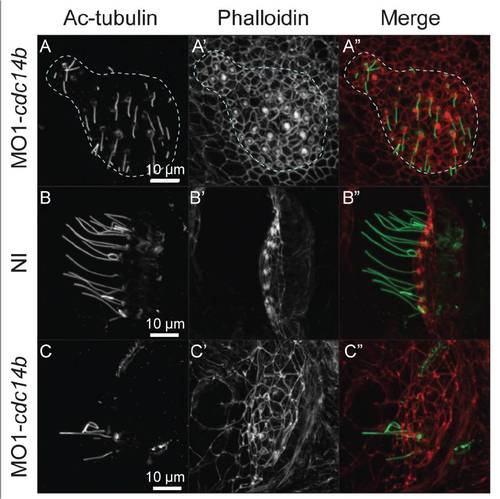
Analysis of Cdc14B function during ciliogenesis. (A) Dynamic distribution of Cdc14B during cell division at 8 hpf. The embryo was injected with synthetic cdc14b_tv1-GFP (green) and centrin-cherry (red) RNAs. Dashed lines outline a cell in interphase. (Top cell) A dividing cell proceeding through anaphase (00:00-00:30), telophase (1:00-2:00) and interphase (3:00-3:30). Arrowheads indicate the centrioles. Dotted lines outline the nuclear envelope. (B,C) Positioning of the centrioles in the posterior kidney duct at 27 hpf in non-injected (B) and MO1-cdc14b-injected (C) embryos visualized with an anti-γ-tubulin antibody (red) and phalloidin (green). |
|
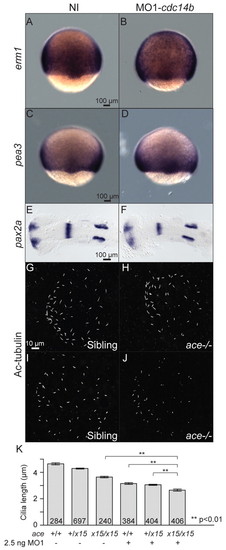
Interaction between the FGF pathway and Cdc14B. Expression pattern of erm1 (A,B) and pea3 (C,D) at 8 hpf, and pax2a (E,F) at 17 hpf. (G-J) KV cilia in siblings and fgf8/acex15 homozygous mutant embryos, non-injected (G,H) or injected with MO1-cdc14b (I,J) at 14 hpf visualized with an anti-Ac-tubulin antibody. (K) Cilia length was determined at 14 hpf. The numbers of cilia analyzed are indicated. Data are mean ± s.e.m. Statistics were carried out using Student′s t-test. EXPRESSION / LABELING:
PHENOTYPE:
|
|
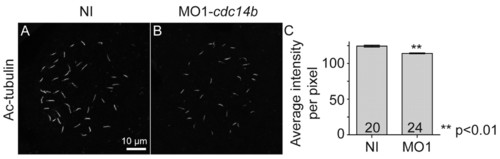
Cdc14B function in cilia length regulation. (A,B) KV cilia at 14 hpf visualized with an anti-Ac-tubulin antibody. (C) Quantification of the acetylation level in the microtubules of KV cilia in non-injected and MO1-cdc14b-injected embryos at 14 hpf. Data are mean ± s.e.m. Statistics were carried out using Student′s t-test. PHENOTYPE:
|
|
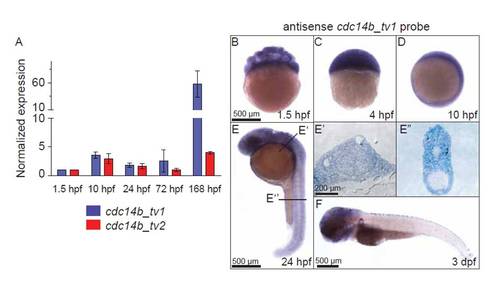
Expression pattern of cdc14b. (A) Quantitative RT-PCR comparing cdc14b_tv1 and cdc14b_tv2 isoforms level of expression at specific times during embryonic and early larval development. Data are represented as mean±s.e.m. Statistics were carried out using Student′s t-test. (B-F) In situ hybridization with antisense cdc14b_tv1 probe. (E′,E′′) Sections stained with Toluidine Blue through a 24 hpf embryo at locations indicated in E. |
|
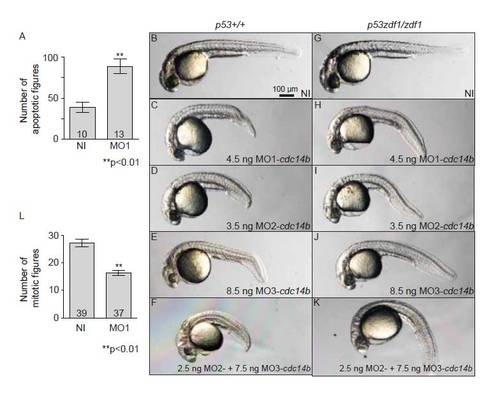
Cdc14B loss-of-function effect on cell death and cell proliferation. (A) Number of apoptotic figures in the tail of non-injected or MO1-cdc14b-injected embryos at 27 hpf. (B-K) NI and MO1-cdc14b injected embryos at 31 hpf in tp53+/+ (B-F) and tp53zdf1/zdf1 homozygous mutant (G-K) background. (L) Number of mitotic figures in the tail of non-injected or MO1-cdc14b injected embryos at 27 hpf. The numbers of embryos analyzed are indicated. Data are represented as mean±s.e.m. Statistics were carried out using Student′s t-test. |
|
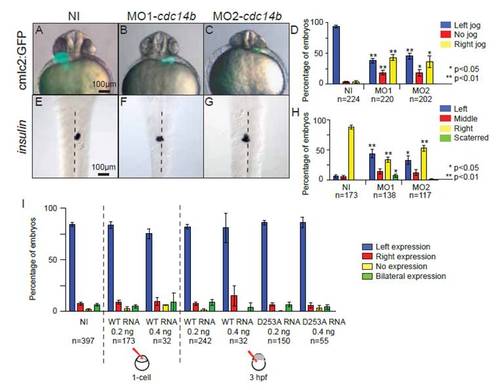
Cdc14B loss-of-function effect on LR asymmetry. (A-D) Compound image and LR asymmetry quantification of cmlc2:GFP in non-injected and cdc14b MO-injected embryos at 31 hpf. (E-H) In situ hybridization and expression pattern quantification in non-injected and cdc14b MO-injected embryos of insulin at 50 hpf. (I) Quantification of LR asymmetry using spaw expression at 19 hpf in embryos injected with wild-type or mutated (D253A) cdc14b mRNAs. Numbers of embryos are indicated above the lines. Data are represented as mean±s.e.m. Statistics were carried out using Student′s t-test. |
|
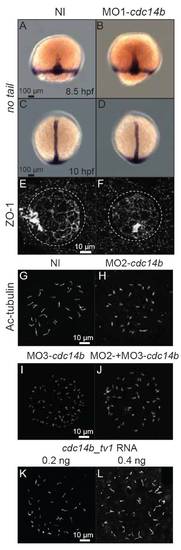
Cdc14B loss- and gain-of-function effects on KV. (A-D) Expression of no tail at 8.5 hpf (A,B) and 10 hpf (C,D). (E,F) KV visualized with an anti-ZO-1 antibody at 14 hpf. (G-L) Cilia in KV of non-injected, cdc14b MO-injected and cdc14b_tv1 overexpressing embryos at 14 hpf visualized with an anti-Ac-tubulin antibody. |
|
Cdc14B loss-of-function effect on other types of cilia. (A) Saccular macula of MO1-cdc14b-injected larvae at 4 dpf. The structure is outlined in blue. (B,C) Posterior crista of non-injected and MO1-cdc14b-injected larvae at 4 dpf. The structures are visualized with phalloidin and anti-Ac-tubulin antibody. |

Unillustrated author statements EXPRESSION / LABELING:
|