- Title
-
A high-throughput chemically induced inflammation assay in zebrafish
- Authors
- d'Alencon, C.A., Pena, O.A., Wittmann, C., Gallardo, V.E., Jones, R.A., Loosli, F., Liebel, U., Grabher, C., and Allende, M.L.
- Source
- Full text @ BMC Biol.
|
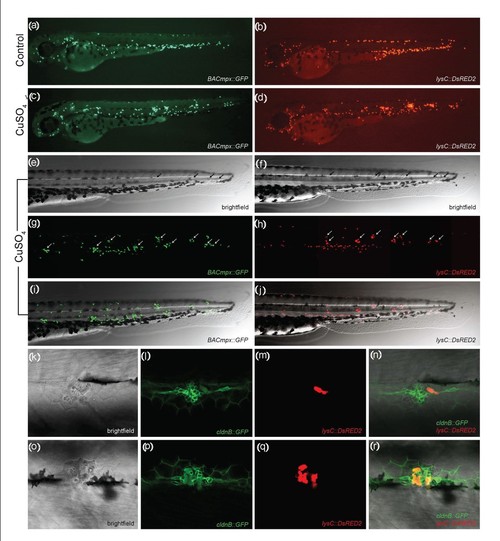
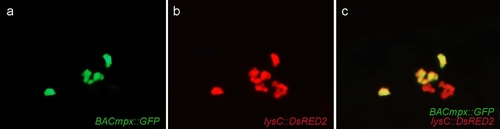
Leukocytes migrate specifically to damaged lateral line neuromasts in zebrafish larvae. (a-j) 56-hours postfertilization (56-hpf) BACmpx::GFP or lysC::DsRED2 transgenic zebrafish larvae exhibit green or red fluorescent leukocytes, respectively. (a and b) Untreated fish show the normal distribution of labeled cells, mostly localized in the ventral trunk and tail. (c and d) In copper-treated siblings, leukocytes become localized preferentially to a few clusters along the horizontal midline of the trunk and tail. (e-j) A detailed view of this region in copper-treated animals shows that while many cells disperse throughout the body, other cells congregate in discrete clusters (arrows); no overt tissue damage to the larvae is observed in bright-field images. (k-r) A mating cross of cldnB::GFP and lysC::DsRED2 transgenic fish labels neuromasts in green and leukocytes in red. Posterior trunk neuromasts were imaged immediately after adding copper (k-n) or 20 minutes after copper treatment (o-r) using bright-field red or green fluorescence illumination. Few, if any, leukocytes are seen near neuromasts at the beginning of treatment. (m and n) Here a case where a single leukocyte is present is shown. (q and r) In contrast, copper-treated fish have numerous red fluorescent leukocytes interspersed within the neuromast cells. Note the extent of damage induced by copper in the neuromast cells (compare Figures 1l and 1p). |
|
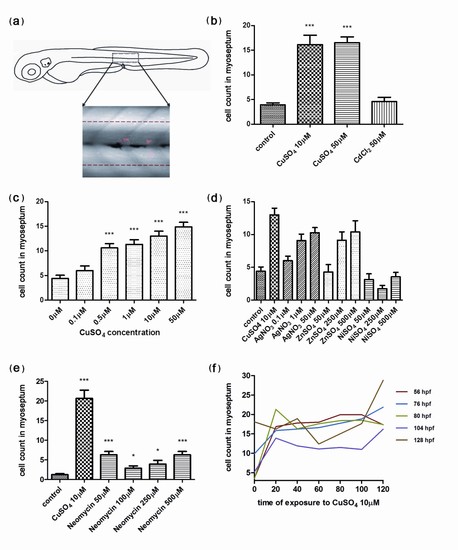
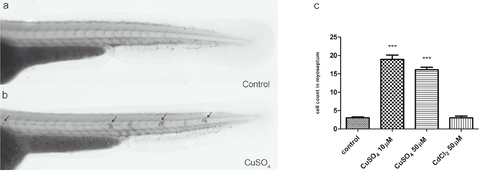
Quantification of infiltrating leukocytes in the lateral line after diverse treatments: the chemically induced inflammation (ChIn) assay. (a) Schematic view of a 3 days postfertilization (dpf) larva. The boxed area corresponds to the horizontal myoseptum (line). An area of approximately 10 cell diameters is delimited around the myoseptum (dotted red lines) and corresponds to the area where leukocytes were counted in all manual quantification experiments. (b) Significant induction of leukocyte recruitment to the lateral line by copper treatment. The graph shows average leukocyte numbers in the lateral line in negative controls (untreated fish or cadmium chloride-treated fish) and in copper-treated fish. (c) The effect of copper on leukocyte recruitment to the lateral line is concentration dependent. (d) Effectiveness of other metals in the ChIn assay. (e) Neomycin, at concentrations that eliminate hair cells, also induces leukocyte recruitment, but less effectively than copper. (f) Larvae of different ages, from 56 to 128 hpf, were exposed to 10 μM CuSO4 and were monitored for leukocytes present at the myoseptum every 20 minutes thereafter until 120 minutes. Fish at all stages analyzed showed similar behaviors and exhibited increased presence of leukocytes at the lateral line by 20 minutes after initiating exposure to copper. For all experiments, at least 15 larvae were used for each condition. ***P < 0.001. |
|
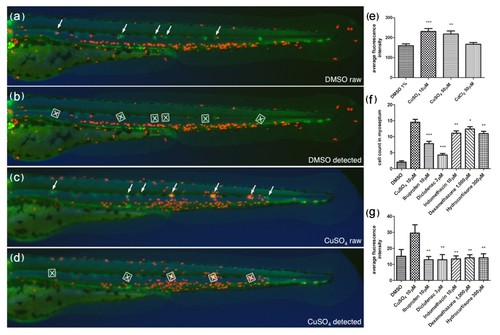
Automated ChIn assay. (a-d) Image acquisition method using compound transgenic larvae cldnB::GFP and lysC::DsRED2. Images show control (DMSO) (a and b) and treated (CuSO4) (c and d) fish revealing neuromasts (green, arrows) and leukocytes (red). Shown are the raw images (a and c) and the number and identity of the neuromasts that were automatically detected by the software (b and d) (white squares). The image analysis software determines the average red fluorescence intensity per square (neuromast area) and calculates the data averaged for all squares detected within one larva. Note that the program is able to detect most, but not all, of the visible neuromasts. The variable neuromast detection success is compensated by using more larvae than in the manual method: 24 per plate, in triplicate, averaging around 50 data-producing larvae per condition. (e) A control experiment using the automated ChIn assay. Untreated or metal-exposed double-transgenic fish were imaged, and red fluorescence was averaged from three experiments. Results are comparable to manual ChIn assays. (f and g) Comparison of ChIn assay results between the manual quantification method (f) and automated detection (g) of anti-inflammatory drug activity. |
|
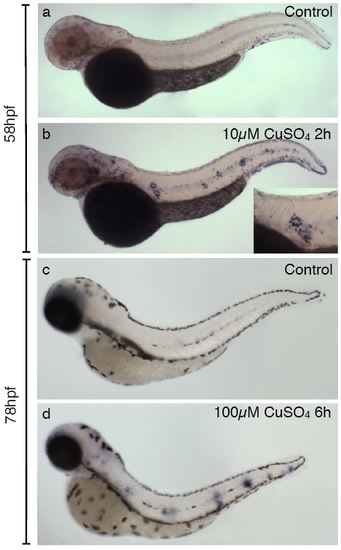
Induction of matrix metalloproteinase 9 (mmp9) expression by copper treatment in zebrafish larvae. In situ hybridization to detect expression of mmp9 was carried out in control (a and c) and sibling fish treated with copper sulfate (c and d). (a and b) Induction of mmp9 expression after treating 2-day-old fish with 10 μM CuSO4 for 40 minutes. While control fish have few detectable cells labeled with probe, clusters of highly labeled cells are seen in a characteristic pattern along the flanks of the treated animals. The inset shows a closeup image of one of these clusters. (c and d) mmp9 induction by treatment of 3-day-old fish with 100 μM CuSO4 for 6 hours and fixed immediately thereafter. Note that clustering of labeled cells at discrete positions along the midline is also apparent after the more severe treatment. |
|
Neutrophils and macrophages behave similarly in response to copper exposure. Compound BACmpx::GFP/lysC::DsRED2 transgenic fish were treated with 10 μM copper sulphate, and the area surrounding a neuromast was imaged 20 minutes after initiation of exposure. Detection of cells was carried out in the GFP channel (a) and the red (DsRED2) channel (b), and both images were merged (c). Both neutrophils (yellow cells in (c)) and macrophages (red cells in (c)) can be observed to migrate to damaged neuromasts. |
|
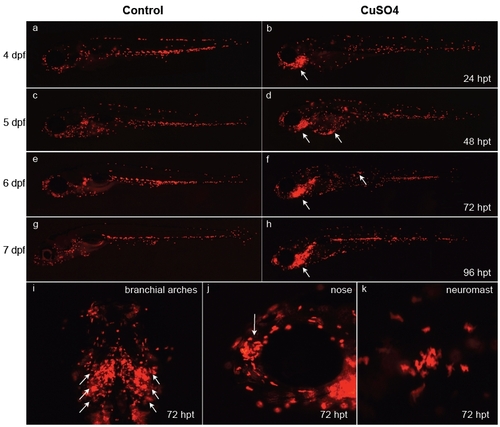
Behavior of leukocytes after long-term copper exposure in zebrafish larvae. At 3 days postfertilization (dpf), transgenic lysC::DsRED2 fish were left untreated (a, c, e, and g) or exposed permanently thereafter to 10 μM CuSO4 (b, d, f, h, and i-k) and were imaged daily until 7 dpf (times in the right-hand column are expressed in hours posttreatment, hpt). (a-h) Lateral views of entire larvae. Note the general dispersal of leukocytes in treated vs. control fish simultaneous with accumulation in different anterior regions, especially the branchial arches of the animal beginning 1 day after beginning treatment. (i-k) Closeups of specific areas at 72 hpt. (i) Ventral view of branchial arches. (j) Lateral view of head; arrow indicates olfactory pit area. Closeup view of the area surrounding a neuromast. Note that fish exposed for long periods to copper sulfate suffer developmental delays. |
|
Chemically induced inflammation assay (ChIn) using Sudan Black. (a and b) Bright-field images of untreated (a) and 10 μM CuSO4-treated (b) 56-hpf casper larvae stained with Sudan Black to reveal leukocytes. Note the congregation of labeled cells at the posterior lateral line neuromasts (arrows). (c) Quantification of leukocyte migration (detected by Sudan Black staining) to the lateral line in untreated and metal-exposed larvae. The result is equivalent to that obtained with BACmpx::GFP or lysC::DsRED2 transgenic larvae. |
|
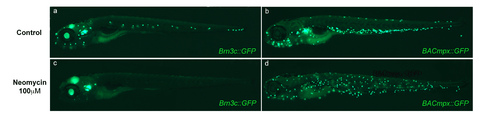
Neomycin ablates hair cells but fails to induce localization of leukocytes to the horizontal myoseptum. (a and c) Transgenic Brn3c::mGFP larvae express GFP in hair cells of the lateral line neuromasts as well as in cells of the ear, eye and brain. Transgenic larvae were left untreated (a) or were treated with 100 μM neomycin for 2 hours (b) and imaged under fluorescence. Note the ablation of lateral line hair cells, though other expressing tissues are unaffected. (b and d) BACmpx::GFP larvae were treated in parallel with Brn3c::GFP larvae to examine leukocyte behavior. Note that while leukocytes disperse with the neomycin treatment, they do not congregate near neuromasts as they do with copper treatment. |