- Title
-
Oda16/Wdr69 is essential for axonemal dynein assembly and ciliary motility during zebrafish embryogenesis
- Authors
- Gao, C., Wang, G., Amack, J.D., and Mitchell, D.R.
- Source
- Full text @ Dev. Dyn.
|
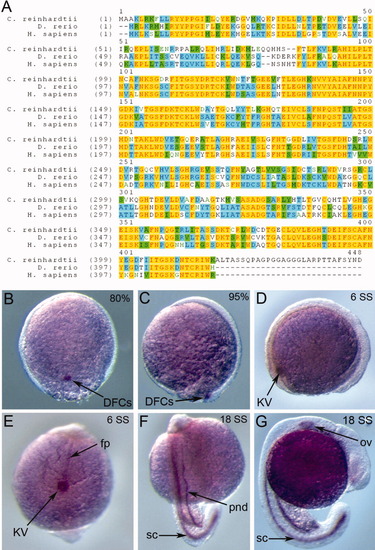
Zebrafish wdr69 is expressed in cells that assemble motile cilia. A: Protein sequence alignment of Oda16 homologs from Chlamydomonas reinhardtii, Danio renio, and Homo sapiens. Yellow color highlights conserved residues among the three species, blue color highlights residues conserved in two species and green color highlights conservative replacements. B-G: RNA in situ hybridizations detecting wdr69 expression during early zebrafish development. B,C: At the 80% (B) and 95% (C) epiboly stages, wdr69 is expressed exclusively in dorsal forerunner cells (DFCs). D,E: At the six somite stage (SS), wdr69 is expressed in Kupffer′s vesicle (KV) (D, E) and the floorplate (fp) of the neural tube (E). F,G: At 18 SS, wdr69 is expressed in the spinal cord (sc), pronephric ducts (pnd) (F) and otic vesicle (ov) (G). EXPRESSION / LABELING:
|
|
wdr69 morpholino oligonucleotides (MO) knockdown embryos develop phenotypes associated with defects in ciliary motility. A-C: Live embryos at 2 days postfertilization (dpf). Control MO injected embryos exhibited normal morphology (A), whereas wdr69 MOAUG morphant embryos developed phenotypes associated with cilia defects, including a curled tail (B), kidney cysts (arrow in C) and pericardial edema (arrowhead in C). D,E: Otoliths in otic vesicles at 2 dpf. Control MO injected embryos developed two correctly positioned otoliths (arrows in D). wdr69 MOAUG morphants often developed a third ectopically positioned otolith (red arrow in E). F:wdr69 MOI2E3 morphants developed the same phenotypes as wdr69 MOAUG morphants (this embryo was treated with PTU to inhibit melanin biosythesis for RNA in situ analysis). G: RT-PCR analysis shows wdr69 MOI2E3 causes mis-splicing of wdr69 transcripts. A normally spliced cDNA containing exons 2-4 was detected in control MO injected embryos. The level of this cDNA was reduced in wdr69 MOI2E3 morphants and a smaller fragment lacking exon 3 was observed in wdr69 MOI2E3 morphants. β-actin was amplified as a loading control. PHENOTYPE:
|
|
Wdr69 is important for the establishment of left-right asymmetry. A-F: Analysis of organ left-right asymmetry by RNA in situ hybridizations using the heart marker cmlc2 (A-C) and gut marker foxa3 (D-F). Control morphants showed normal rightward looping of the heart (arrow in A) and left-sided orientation of the liver (arrow in D). In wdr69 MOAUG morphants, heart looping was often reversed (arrow in B) or linear along the midline (arrow in C). Similarly, liver position in wdr69 MOAUG morphants was frequently reversed (arrow in E) or bilateral (arrows in F). G-I: RNA in situ hybridizations detecting southpaw (spaw) expression in lateral plate mesoderm (LPM) at 18 somite stage (SS; G). spaw expression was restricted to left LPM cells in control morphants (arrow in G), whereas wdr69 MOAUG morphants displayed abnormal right (arrow in H) and bilateral (arrows in I) distributions of spaw expression. J: The percentage of embryos injected with control MO (n = 130), wdr69 MOAUG (n = 67) or wdr69 MOI2E3 (n = 75) showing laterality defects in the heart and gut (see also Table 1). MO, morpholino oligonucleotides (MO). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cilia length and number is not changed in wdr69 morphants. A-F: Cilia were visualized by immunofluorescent staining with acetylated tubulin antibodies. Cilia in embryos injected with wdr69 MOAUG appeared similar to cilia injected with control MO in Kupffer′s vesicle (KV; A,B), pronephric ducts (C,D) and otic vesicles (arrows in E,F point to motile tether cilia). All red scale bars represent 10 μM. G,H: Measurements of KV cilia at six to eight somite stage (SS) showed no significant differences in the average length (G) or number (H) between wdr69 MOAUG (n = 17) and control (n = 21) morphants. Error bars = one standard deviation. MO, morpholino oligonucleotides. EXPRESSION / LABELING:
PHENOTYPE:
|
|
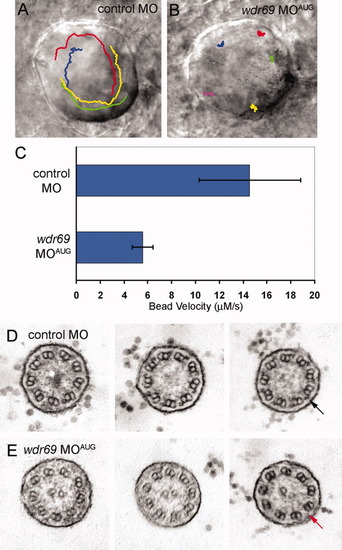
Wdr69 is needed for ciliary motility and outer arm dynein assembly. A,B: Tracking software was used to follow the flow of fluorescent beads injected into Kupffer′s vesicle (KV) of live embryos. Tracks are superimposed on images of KV. A: In control morphants, beads flowed in a circular counterclockwise direction. B: This directional flow was lost in wdr69 MOAUG morphants, where beads bounced around randomly. C: Velocity of bead movement was significantly reduced in wdr69 knockdown embryos (n = 25 beads from 5 embryos) relative to controls (n = 28 beads from 6 embryos). Error bars=one standard deviation. D,E: Electron microscopy of KV cilia. D: In control MO injected embryos, typical outer dynein arms (arrow) were present on all nine outer doublets of all cilia examined. E: In contrast, KV cilia from wdr69 MOAUG morphants displayed greatly reduced numbers of outer dynein arms. MO, morpholino oligonucleotides. PHENOTYPE:
|