- Title
-
The zebrafish flotte lotte mutant reveals that the local retinal environment promotes the differentiation of proliferating precursors emerging from their stem cell niche
- Authors
- Cerveny, K.L., Cavodeassi, F., Turner, K.J., de Jong-Curtain, T.A., Heath, J.K., and Wilson, S.W.
- Source
- Full text @ Development
|
flo mutants have small eyes. (A,B) Lateral (left) and dorsal (right) views of live wild-type (A) and flo (B) zebrafish embryos at 3 dpf. (C-F) Frontal transverse sections through wild-type (C,E) and flo (D,F) embryos at 2 dpf (C,D) and 3 dpf (E,F). (G-J) Frontal cryosections of wild-type (G,I) and flo (H,J) eyes immunostained to detect markers for polarity (G,H, γ-tubulin, green) and differentiation [I,J; zn5 (Alcama), RGCs, red; HuC::GFP (Elavl3), RGCs and amacrine cells, green]. rgcl, retinal ganglion cell layer; inl, inner nuclear layer; onl, outer nuclear layer; rpe, retinal pigmented epithelium; cmz, ciliary marginal zone; ipl, inner plexiform layer; opl, outer plexiform layer. |
|
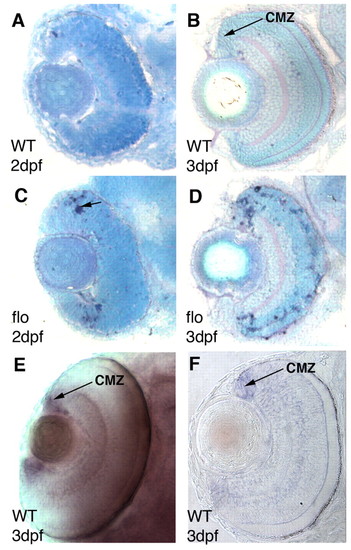
elys is expressed in the CMZ and central CMZ cells apoptose in flo mutants. (A-D) Frontal plastic sections of wild-type (A,B) and flo (C,D) zebrafish eyes at 2 dpf (A,C) and 3 dpf (B,D). TUNEL+ apoptotic cells are present at the central limit of the CMZ in the flo retina (dark blue nuclei, arrow in C). (E,F) Whole-mount eye (E) and frontal plastic section (F) showing that at 3 dpf, elys expression is restricted to the CMZ. |
|
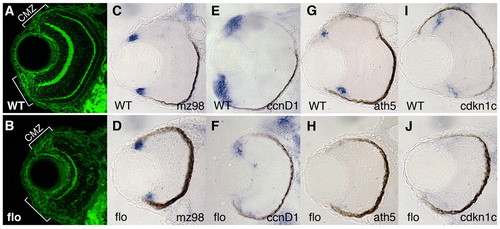
CMZ cells in flo embryos show reduced expression of cycling and differentiation markers. (A,B) CMZ cells are present in flo retinas. Frontal transverse cryosections of zebrafish retinae stained for β-catenin (green). Brackets denote CMZ boundaries. (C-J) Gene expression in subpopulations of cells within the CMZ. mz98, a marker of the peripheral, putative stem cell compartment, is expressed in wild-type siblings (C) and flo (D) embryos. ccnd1 is highly expressed in the wild-type proliferating progenitors of the CMZ (E) but is reduced in flo eyes (F). ath5 (G,H) and cdkn1c (I,J) are expressed in cells in central CMZ concomitant with the final division cycle in the wild-type CMZ (G,I), but are absent in the flo retina (H,J). |
|
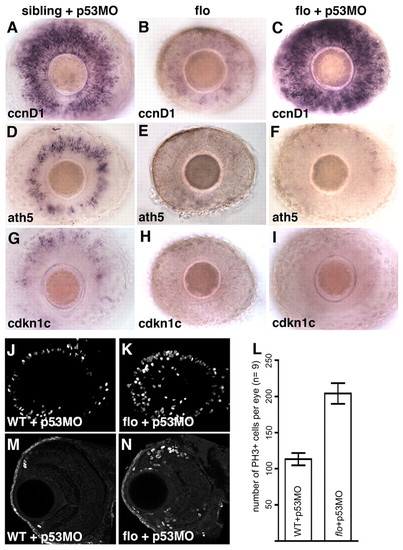
flo CMZ cells fail to transition from proliferation to differentiation, even in the absence of apoptosis. (A-I) Intact retinae from 3-dpf wild-type (A,D,G) and flo (B,E,H) zebrafish embryos and flo embryos injected with p53MO (C,F,I), labelled to show expression of the genes indicated bottom left. flo CMZ cells robustly express the proliferative marker ccnd1 (C) but not markers of cell cycle exit (F,I) when p53-mediated apoptosis is inhibited. (J,K,M,N) Confocal projections of laterally viewed wild-type + p53MO (J) and flo + p53MO (K) whole retinae and coronal sections of wild-type + p53MO (M) and flo + p53MO (N) retinae, all stained with anti-phosphohistone H3 (PH3). flo + p53MO eyes exhibit increased proliferation in/near the CMZ. (L) flo + p53MO embryos contain significantly more mitotic cells than wild-type + p53MO siblings (P<0.0001, Student′s t-test). The average number of mitotic retinal cells per eye (n=9 eyes from 9 different fish, examples shown in J,K) was calculated and graphed with error bars (95% confidence limits). |
|
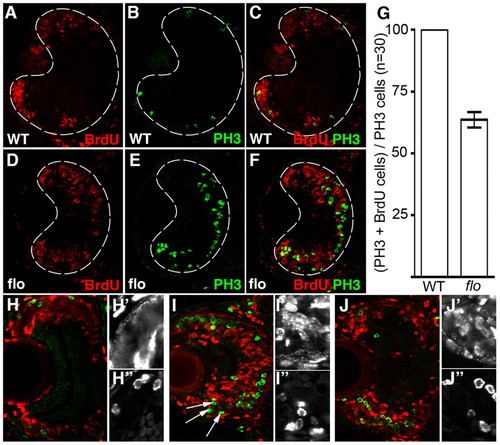
flo retinal cells fail to progress efficiently through the cell cycle. (A-F,H-J″) Frontal transverse cryosections of zebrafish retina stained for markers of DNA replication (BrdU, red) and M phase (PH3, green). Wild-type (A-C) and flo + p53MO (D-F) embryos were injected with BrdU at 53 hpf and again at 55 hpf, then fixed <75 minutes later. Single-channel magnifications of BrdU (H′,I′,J′) and PH3 (H″,I″,J″) in the CMZ are shown. (G) The average number of PH3+ cells that are also BrdU+ per section (n=30 eyes) was calculated and graphed with error bars (99% confidence limits). All of the PH3+ cells in wild-type siblings were also BrdU+. Significantly fewer cells (27.5%) were double labelled in flo embryos (P<0.0001, Student′s t-test). As above, wild-type (H), flo (I) and flo + p53MO (J) embryos were injected with BrdU but then fixed 24 hours later. |
|
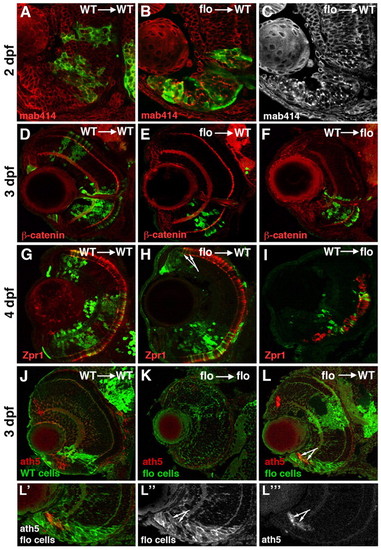
flo cells survive and differentiate in a wild-type environment. (A-L″′) Frontal sections of zebrafish eyes at various ages labelled with antibodies as indicated bottom left; mab414 recognises the Nup107-160 subcomplex of nuclear pores, β-catenin highlights cell membranes and plexiform layers; zpr1 recognises cone photoreceptors. GFP-labelled cells from either wild-type (A,D,F,G,I,J) or flo (B,C,E,H,K,L) donor embryos were transplanted into wild-type (A-E,G,H,J,L) or flo (F,I,K) hosts. The arrows in H and L point to flo mutant photoreceptors and ath5-expressing cells emerging from the CMZ in wild-type eyes, respectively. See text for the specific numbers of mosaic eyes examined for each condition. ath5 expression (or lack thereof) in wild-type (J) and flo (K) retinae is shown for reference. (L′,L″,L″′) Single-channel magnifications from L illustrate flo CMZ cells in a wild-type environment. |
|
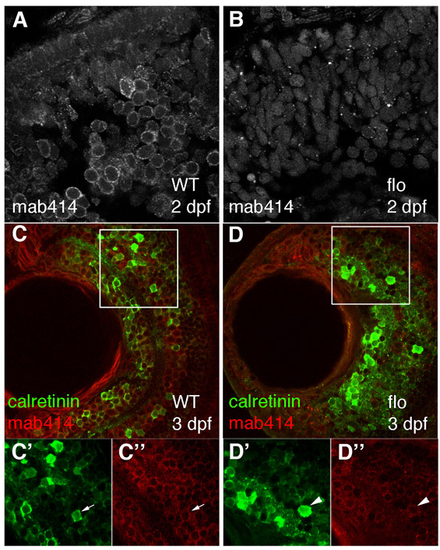
Retinal cells in flo embryos contain disrupted nuclear pores, but still express a marker of neuronal differentiation and function. (A-D″) Nuclear pores and calretinin-positive cells were visualised in wild-type (A,C) and flo (B,D) retinae from 3 dpf by staining with mab414 (grey in A,B, red in C,D) and calretinin (green) sera. C′-D″ are enlargements of the single channels from the boxed regions in C and D, respectively. Calretinin-positive cells (green) from approximately the same retinal position have nuclei ringed uniformly by red mab414 staining (WT, C′,C″, arrow) or have aberrant nuclear pores (flo, D′,D″, arrowhead). |
|
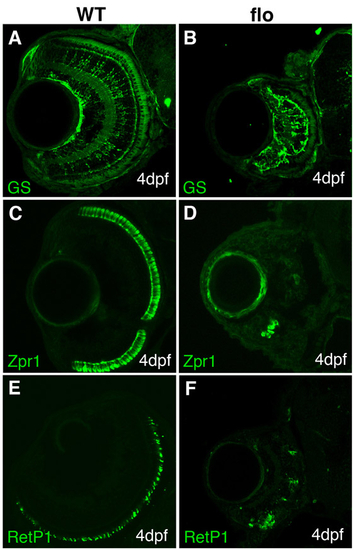
Markers for glial and photoreceptor differentiation are present but reduced in flo eyes. (A-F) Frontal cryosections of 4-dpf embryos immunostained to detect later-born cell types. Glutamine synthase (GS) is found predominantly in Müller glia (A,B), zpr1 stains cones (C,D) and RetP1 highlights rods (E,F). |
|
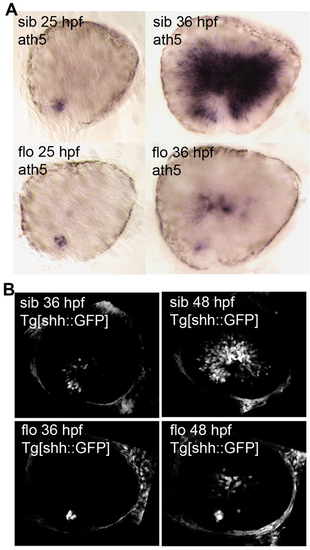
Signals required for neurogenesis in the early differentiating central retina are delayed and reduced in flo eyes. (A) Lateral views of dissected eyes revealing the stereotypical spatial and temporal expression pattern of ath5 (top) that presages the generation of the first-born retinal neurons: the RGCs. This pattern is delayed and reduced in flo mutants (bottom). (B) Lateral confocal projections of live wild-type and flo embryos expressing GFP from the shh promoter showing the spread of Shh signal throughout the central retina as neurogenesis proceeds. |
|
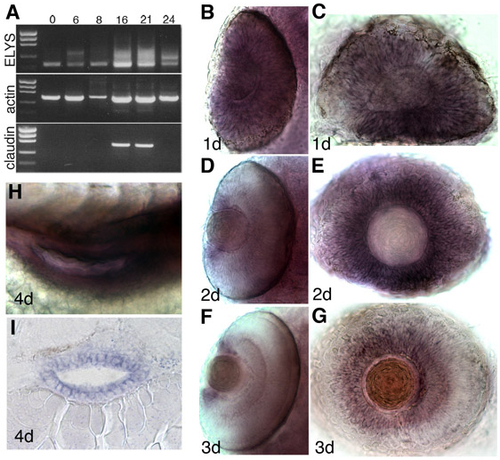
elys mRNA is maternally inherited, initially expressed ubiquitously, and later restricted to tissues with high levels of proliferation. (A) RT-PCR of the first two exons of elys and the entire open reading frame of both actin and claudin a. Numbers above each lane designate hpf. (B-G) elys expression in the eye. At 1 dpf, elys is expressed throughout the retina (B,C), but by 2 and 3 dpf its expression is restricted to the CMZ (D-G). Whole-mount dorsal views (B,D,F) and lateral views of dissected eyes (C,E,G). (H,I) elys is also strongly expressed in the developing intestine. (H) Lateral view, whole-mount; (I) frontal cross-section. |
|
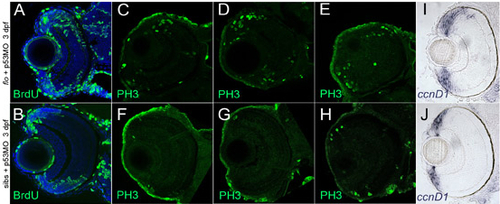
flo retinae exhibit increased proliferation in/near the CMZ. (A-H) Markers for the S (BrdU) and M (PH3) phases of the cell cycle are elevated in flo + p53MO embryos at 3 dpf. Frontal transverse cryosections stained with anti-BrdU (A,B, green) or anti-PH3 (C-H, green) to identify proliferating (A,B) or mitotic (C-H) cells. Nuclei in A,B are counterstained with To-Pro 633 (blue). (C-H) Of the sections examined (n=10 eyes, three representative examples of each are shown), WT retinae contained 2-8 PH3+ cells near the periphery, whereas the number of PH3+ cells in flo retinae ranged from 16-32. (I,J) Additionally, the region of the CMZ marked by cyclin D1 expression (ccnd1) is noticeably expanded when apoptosis is blocked in flo embryos. |
|
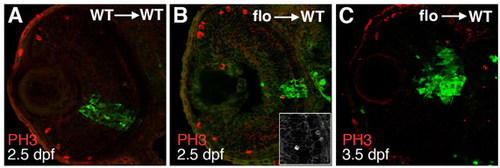
flo cells exit the cell cycle in wild-type eyes. (A-C) GFP-labelled cells were transplanted from wild-type (A) or flo (B,C) donor embryos into wild-type hosts, allowed to develop until 2.5 dpf (A,B) or 3.5 dpf (C) and then analysed for the presence of PH3+ (dividing) cells (red) in the transplanted clones (green). |