- Title
-
Biogenesis of GPI-anchored proteins is essential for surface expression of sodium channels in zebrafish Rohon-Beard neurons to respond to mechanosensory stimulation
- Authors
- Nakano, Y., Fujita, M., Ogino, K., Saint-Amant, L., Kinoshita, T., Oda, Y., and Hirata, H.
- Source
- Full text @ Development
|
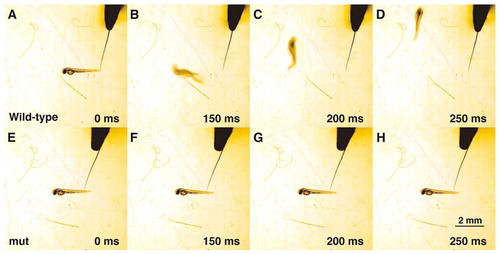
mi310 embryos do not respond to touch. (A-D) Mechanosensory stimulation induced a wild-type embryo [2 days post-fertilization (dpf)] to swim away rapidly. (E-H) A mi310 embryo (2 dpf) did not respond to touch. |
|
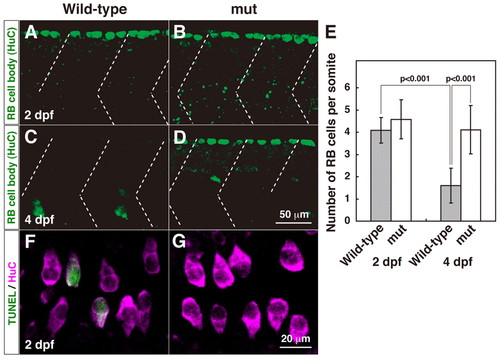
Programmed cell death of RB neurons is diminished in mi310 mutants. (A) In a lateral view of a wild-type embryo at 2 dpf, anti-HuC labeled large cell bodies of RB neurons (green). Dashed lines represent somite boundaries. (B) A mutant embryo displayed a comparable number of HuC-positive cells at 2 dpf. (C) In wild type, RB neurons were eliminated by 4 dpf. (D) HuC-positive RB cells were observed in mutant larva at 4 dpf. (E) The number of RB neurons is significantly reduced between 2 and 4 dpf in wild type but not in mutants. (F) In a dorsal view of a wild-type embryo, some HuC-positive neurons (magenta) were also positive for TUNEL (green) at 2 dpf. (G) HuC-TUNEL double-positive neurons were rarely observed in mutants. |
|
Mutant RB neurons fail to generate normal action potentials owing to defective sodium currents. (A) Injection of step currents up to 100 pA elicited typically large action potentials in a wild-type RB neuron. (B) Current injection into a mutant RB cell failed to generate normal action potentials. (C) The spikes in wild-type RB neurons were eliminated by bath application of TTX, a sodium channel blocker. (D) The small, partial spikes in mutant RB neurons were also eliminated by TTX. (E) Inward sodium currents elicited by voltage steps from -100 mV to 0 mV were recorded in wild-type RB neurons. (F) Inward sodium currents in mutant RB neurons were observed but with smaller amplitude than those seen in wild-type neurons. (G) The inward sodium currents in wild type were blocked in the presence of TTX. (H) The sodium currents in mutant RB cells were also eliminated by TTX. PHENOTYPE:
|
|
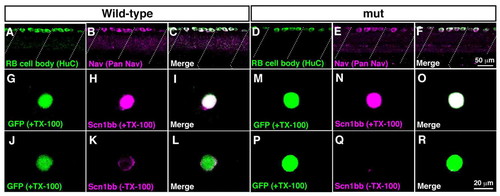
Surface expression of sodium channels is impaired in mutant RB neurons. (A-F) Wholemount immunostaining showed Nav expression in wild-type and mutant RB neurons at 2 dpf. (A) Anti-HuC labeled cell bodies of wild-type RB neurons at the dorsal spinal cord. Dotted lines represent somite boundaries. (B) Immunostaining with anti-pan Nav displayed Nav expression in wild-type spinal cord. (C) A merged image showed that all HuC-positive RB cells expressed Nav in wild-type. (D) Labeling with anti-HuC showed RB neurons in mutant spinal cord. (E) Anti-pan Nav labeled dorsally located spinal neurons in mutants. (F) All of the HuC-positive mutant RB neurons expressed Nav. (G-R) Dissociated RB neurons (GFP-positive) from 2 dpf embryos were labeled with anti-Scn1bb, with or without TX-100, which permeabilizes the plasma membrane. Note that anti-Scn1bb reacts with the extracellular domain of Scn1bb. (G-I) Scn1bb staining in the permeable condition (+TX-100) demonstrated Scn1bb expression in a wild-type RB neuron. (J-L) Labeling of Scn1bb in the impermeable condition (-TX-100) displayed surface distribution of Scn1bb in a wild-type RB cell. (M-O) Scn1bb staining with TX-100 showed Scn1bb expression in a wild-type RB neuron. (P-R) In the absence of TX-100, the surface labeling of Scn1bb was missing in a mutant RB cell, suggesting that Scn1bb exists inside the mutant cell without expression at the surface. |
|
Surface distribution of Scn1bb in RB neurons is diminished by knocking down GPI transamidase subunits. Dissociated RB neurons (GFP-positive) from 2 dpf embryos were labeled with anti-Scn1bb in the permeable (+TX-100) or impermeable (-TX-100) condition. (A-F) In control morpholino (MO)-injection, anti-Scn1bb labeled a whole RB cell in the presence of TX-100 (A-C), whereas it labeled the circumference of an RB cell in the absence of TX-100 (D-F). (G-I) Labeling with anti-Scn1bb with TX-100 displayed Scn1bb expression in an RB cell dissociated from a pigu morphant. (J-L) Scn1bb staining without TX-100 represented the impairment of Scn1bb surface distribution in a pigu morphant RB neuron. (M-O) Scn1bb labeling with TX-100 showed Scn1bb expression in a gpi8 morphant RB cell. (P-R) The surface expression of Scn1bb was not seen in an RB cell dissociated from a gpi8 morphant. |
|
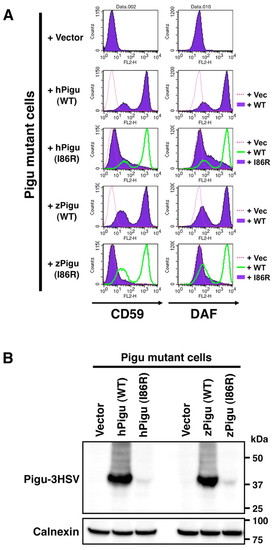
Wild-type Pigu restores the surface expression of GPI-anchored proteins in Pigu-deficient CHO cells. (A) Control or Pigu expression vectors were transfected into Pigu mutant CHO cells and the surface expressions of GPI-anchored proteins (CD59 and DAF) were estimated by flow cytometry. Surface expressions of CD59 and DAF were lower in vector-transfected Pigu mutant cells. Following transfection of human wild-type PIGU or zebrafish wild-type Pigu, surface expressions of CD59 and DAF were increased, showing that GPI transamidase activity was restored by expression of wild-type Pigu. Recovery of surface distribution of GPI-anchored proteins was not seen when transfected with human mutant (I86R) PIGU or zebrafish mutant (I86R) Pigu constructs. (B) The I86R mutation in Pigu destabilized the protein. Whole cell extracts from each pool of the transfected CHO cells were probed with anti-HSV to examine Pigu protein levels. Compared with human and zebrafish wild-type Pigu expressions, the protein levels of human PIGU (I86R) and zebrafish PIGU (I86R) were significantly lower. Calnexin is used as a loading control. |
|
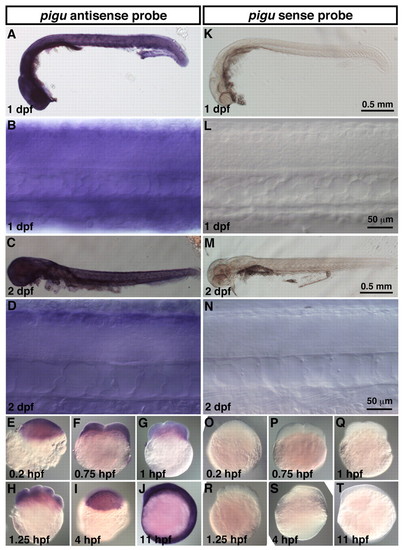
pigu is expressed ubiquitously by whole embryos. In situ hybridization with pigu antisense (A-J) or sense probes (K-T). Wholemounts and DIC images at the spinal cord showed that pigu is expressed by whole zebrafish embryos at 1 dpf (A,B) and 2 dpf (C,D). Expression of pigu mRNA was also observed at 0.2 hpf (1-cell stage; E), 0.75 hpf (2-cell stage; F), 1 hpf (4-cell stage; G), 1.25 hpf (8-cell stage; H), 4 hpf (sphere stage; I) and 11 hpf (early segmentation stage; J). Staining with sense probe displayed no pigu expression at any stages (K-T). EXPRESSION / LABELING:
|
|
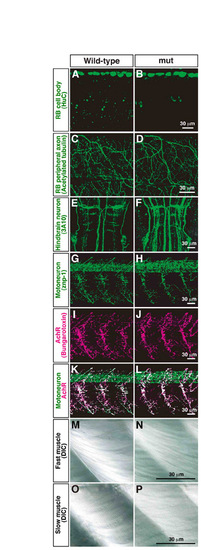
Morphology of RB neurons, CNS neurons and muscle is normal in mi310 mutants. (A-D) Anti-HuC and anti-acetylated tubulin labeled cell bodies and peripheral axons of RB neurons, respectively, in both wild-type (A,C) and mutant (B,D) embryos. (E,F) Reticulospinal neurons in hindbrain were visualized with 3A10 antibody. (G-L) Motoneurons and nicotinic acetylcholine receptors were labeled with znp-1 (G,H) and α-bungarotoxin (I,J), respectively, and their colocalizations represented neuromuscular junctions (K,L). (M-P) Fast muscle fibers (M,N) and slow muscle fibers (O,P) were visualized by DIC in both wild-type and mutant muscle. |
|
Whole-cell patch-clamp recording from RB neurons. (A) Schematic summary of the experimental procedures. Embryos (2 dpf) were pinned on a silicone dish and their skin and muscle were removed. A glass electrode was applied to a GFP-positive RB neuron to record from a single RB cell. (B-E) The patched neurons were identifiable by their morphology. GFP-expressing neurons with large cell bodies located at the dorsal spinal cord were used for whole-cell patch-clamp recording. After whole-cell recording, each patched cell was morphologically identified as an RB neuron. (B) GFP fluorescence. (C) Rhodamine fluorescence diffused from a patch electrode. (D) DIC image. (E) A merged image of GFP and rhodamine confirmed that the patched cell is an RB neuron. Arrows represent patch-clamped RB cells. |

Unillustrated author statements PHENOTYPE:
|