- Title
-
Differential Regulation of Protrusion and Polarity by PI(3)K during Neutrophil Motility in Live Zebrafish
- Authors
- Yoo, S.K., Deng, Q., Cavnar, P.J., Wu, Y.I., Hahn, K.M., and Huttenlocher, A.
- Source
- Full text @ Dev. Cell
|
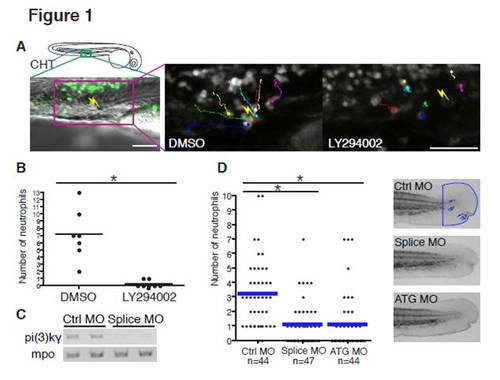
PI(3)Kγ Is Necessary for Directed Migration of Neutrophils In Vivo (A) Sixty-five micromolar LY294002 treatment inhibits attraction of neutrophils to a laser wound in the caudal hematopoietic tissue (CHT). The lines indicate tracking of individual neutrophils over 30 min and the yellow thunder shows the position of the laser wound. Note that neutrophils migrate rapidly toward the wound in control (DMSO, but not after sLY294002 treatment (Movie S1). Scale bar = 50 μm. (B) The number of neutrophils that reach the laser wound in the CHT within 30 min is quantified (seven movies for each condition, *p < 0.001, two-tailed unpaired t test). Note that LY294002 treatment inhibits attraction of neutrophils to the wound. (C) Splice morpholino disturbs splicing of pi(3)kγ transcript. Mpo transcript indicates integrity of neutrophils in morphants. (D) Knockdown of PI(3)Kγ inhibits directional migration of neutrophils to wounds (neutrophils are indicated with blue arrows in control, *p < 0.001, two-tailed unpaired t test). Data of (D) are representative of three separate experiments. PHENOTYPE:
|
|
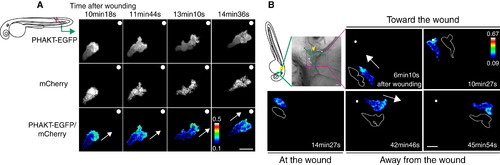
PHAKT-EGFP Translocates to the Leading Edge When Neutrophils Come To and Leave Laser-Induced Wounds (A) Time-lapse ratiometric imaging (PHAKT-EGFP/mCherry) reveals PI(3,4,5)P3-PI(3,4)P2 localization at the leading edge during attraction to a laser wound in the tail fin (>Movie S2A). The white dots and arrows indicate the position of the wound and direction of migration respectively. Scale bar = 10 μm. (B) Reversal of PI(3,4,5)P3-PI(3,4)P2 (ratiometric imaging of PHAKT-EGFP/mCherry) when a neutrophil leaves the laser wound (Movie S2B). Note loss of PI(3,4,5)P3-PI(3,4)P2 polarity at the wound, followed by reversal of polarity to the opposite pole away from the wound when the neutrophil leaves the wound (green line, tracking of a neutrophil; yellow thunder, position of the laser wound; white arrows, direction of migration; illustration with white line, morphology of a neutrophil). Data are representative of more than five time-lapse movies from a minimum of three separate experiments. The numerical values of ratiometric analysis are shown in the scales. Scale bar = 10 μm. |
|
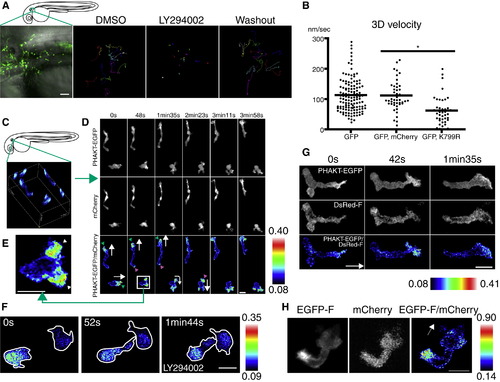
PI(3)K Is Critical for Neutrophil Motility and Is Active at the Leading Edge in the Mesenchymal Tissues of the Head (A) Random migration of neutrophils is arrested by 65 μM LY294002 and restored after washout of the drug. The lines indicate tracking of neutrophil motility (12 cells per condition) imaged for 30 min using Tg(MPO:GFP)uw (Movie S3A). Scale bar = 50 μm. (B) PI(3)K γ K799R disturbs interstitial motility of neutrophils (Movie S3B, *p < 0.001, two-tailed unpaired t test; GFP: 128 neutrophils [40 movies], GFP, mCherry: 45 neutrophils [9 movies], GFP, K799R: 44 neutrophils [34 movies]). (C) Three-dimensional reconstruction of ratiometric image (PHAKT-EGFP/mCherry). (D) Time-lapse ratiometric imaging (PHAKT-EGFP/mCherry) of PI(3,4,5)P3-PI(3,4)P2 dynamics during random migration (Movie S4A). PI(3,4,5)P3-PI(3,4)P2 is mainly localized at the leading edge (green arrowheads) and occasionally at the tail (magenta arrow heads). White arrows indicate direction of migration. Scale bars in (D–H) = 10 μm. (E) PI(3,4,5)P3-PI(3,4)P2 at the bifurcated pseudopod, indicated by arrowheads (Movie S4A). (F) Treatment with 65 μM LY294002 inhibits the leading edge signal of PI(3,4,5)P3-PI(3,4)P2 and induces high ratiometric signals of PHAKT-EGFP/mCherry in the cell body of neutrophils (Movie S4C). Note the rounded tails and thin pseudopods induced by LY294002. (G) PI(3,4,5)P3-PI(3,4)P2 signal at the leading edge by ratiometric imaging of PHAKT-EGFP/farnesylated DsRed (DsRed-F) (Movie S4D). The white arrow indicates direction of migration. (H) Ratiometric imaging of EGFP-F/mCherry reveals periodic accumulation of membrane components at the tail (Movie S4E). The white arrow indicates direction of migration. Images are representative of three (A) and more than five (C–H) time-lapse movies from a minimum of three separate experiments. |
|
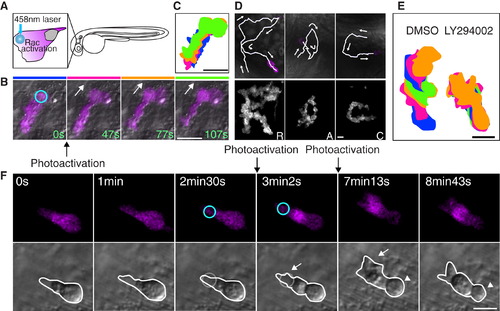
Photoactivation of Rac at the Leading Edge Can Rescue the Protrusion Defects but Not the Rounded Tail or Migration Defects Induced by PI(3)K Inhibition (A) A schematic representation of photoactivation of Rac at the neutrophil leading edge in zebrafish. (B) Photoactivation of Rac at the leading edge induces protrusion and migration of a neutrophil in tissues (Movie S5). The circle indicates the position of Rac photoactivation. Scale bar = 20 μm. (C) Overlayed images of (B) show directional migration induced by photoactivated Rac. Scale bar = 20 μm. (D) Spelling by neutrophil trajectories guided through repetitive photoactivation of Rac at the leading edge (Movie S6). Scale bars in (D–F) = 10 μm. (E) Overlayed images show that PI(3)K inhibition disturbs Rac photoactivation-induced migration. (The leading edge was activated for 20 s twice during 5 min imaging). (F) Photoactivation of Rac at the front (circles) can rescue the protrusion defect induced by PI(3)K inhibition (arrows), but not the rounded tail defect (arrowheads) (Movie S8). Images are representative of more than five time-lapse movies from experiments repeated on at least two separate dates. |
|
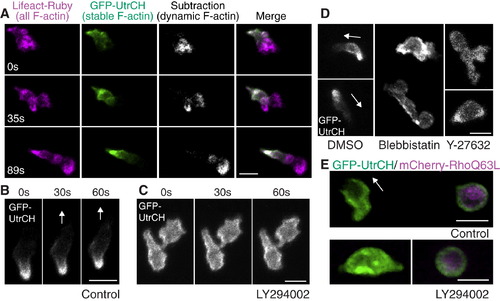
PI(3)K Regulates Anteroposterior Polarity of F-Actin Dynamics (A) Stable F-actin (GFP-UtrCH) is localized at the tail while dynamic F-actin (Lifeact-Ruby subtracted by GFP-UtrCH) is localized at the front (Movie S9). (B) In control, GFP-UtrCH labels the tail. (C) PI(3)K inhibition by LY294002 disturbs tail localization of stable F-actin (Movie S11A). (D) Myosin ATPase and Rho kinase inhibition disturbs tail localization of stable F-actin (Movie S11C). (E) Constitutively active RhoQ63L induces cell rounding and localization of stable F-actin all over the membrane (top panel). PI(3)K inhibition does not relieve constitutively active Rho-mediated effects on cell rounding or localization of stable F-actin (lower panel) (Movie S12A). Images are representative of more than five time-lapse movies from experiments repeated on at least two separate dates. Scale bars = 10 μm. |
|
PI(3)K Regulates Anteroposterior Polarity of F-Actin Dynamics in a Pathway That Is Separable from Rac-Mediated Protrusion (A) In control, photoactivation of Rac at the leading edge induces protrusion at the leading edge with GFP-UtrCH (stable F-actin) localized at the tail (Movie S13A). (B) Photoactivation of Rac with PI(3)K inhibition induces protrusion with GFP-UtrCH (stable F-actin) localized at the leading edge, indicating reverse polarity of F-actin dynamics (Movie S13B). (C) Protrusion induced by photoactivation of Rac induces PHAKT-EGFP accumulation at the leading edge, suggesting a positive feedback from Rac to PI(3,4,5)P3-PI(3,4)P2 gradient (Movie S14). (D) A schematic representation of two-tiered PI(3)K-mediated regulation of cell motility: PI(3)K promotes Rac-mediated actin polymerization at the leading edge while generating anteroposterior polarity of F-actin dynamics. Images are representative of more than five time-lapse movies from experiments repeated on at least two separate dates. Scale bars = 10 μm. |
|
PI(3)Kγ is necessary for directional migration of neutrophils to wounds(related to Figure 1) (A) Larvae at 3 dpf were pretreated with LY294002 or DMSO for 1 hour and wounded in the tail fin with a needle. Neutrophils were stained by Sudan Black staining 1h after wounding. The number of neutrophils in the fin (magenta) and at the wound (green) was counted for each condition. Note that LY294002 inhibits recruitment of neutrophils in a dose-dependent manner. n=33 (DMSO), 39 (32.5 μM LY294002), 38 embryos (65 μM LY294002 ), *, P<0.01, two-tailed unpaired t-test. (B) Larvae at 3dpf were pretreated with AS-605240 or DMSO for 1 hour and wounded in the tail fin with a needle. Neutrophils were stained by Sudan Black staining 1h after wounding. The number of neutrophils in the fin and at the wound was counted for each condition. Inhibition of PI(3)Kγ with AS-605240 significantly inhibits neutrophil attraction to wounds. n=19 (DMSO), 26 (2.5 μM AS-605240), 25 (10 μM AS-605240), *, P<0.01, two-tailed unpaired t-test. (C) Representative images of fluorescent leukocytes in control or PI(3)K morphants (Tg(MPO:mCherry)uw at 2 dpf). These morpholinos do not affect the location or number of neutrophils. |
|
Ratiometric imaging (related to Figure 2) (A) Ratiometric image of EGFP/mCherry. The construct of mCherry was injected into embryos of Tg(MPO:GFP)uw as described in supplementary methods. There is no polarized signal in the bottom right cell expressing both constructs, although the top left cell expressing only EGFP shows high signals in the cell body due to volumetric effects. (B) Reversal of PI(3,4,5)P3-PI(3,4)P2 polarity (ratiometric imaging of PHAKT-EGFP/mCherry) when neutrophils leave the wound induced with a needle in the tail fin (Movie S2C). Tg(MPO:PHAKT-EGFP)uw and Tg(MPO:mCherry)uw were crossed to establish expression of both PHAKT-EGFP and mCherry in neutrophils efficiently. PI(3,4,5)P3-PI(3,4)P2 polarity is established towards the wound during attraction, and the polarity of PI(3,4,5)P3-PI(3,4)P2 is reversed to the opposite pole away from the wound during reverse migration from the wound. White arrows indicate direction of migration. Note high signals of PI(3,4,5)P3-PI(3,4)P2 at the leading edge regardless of the levels of background signals in the cytoplasm. Scale bars, 10 μm. |
|
Imaging ofPI(3,4,5)P3-PI(3,4)P2 (related to Figure 3) (A) PI(3)Kγ K799R expression impairs localization of PHAKT-EGFP at the leading edge, suggesting that PI(3)Kγ is primarily responsible for PI(3,4,5)P3-PI(3,4)P2 gradient formation. (B) Expression of Δp85α, a deletion mutant of the adaptor subunit of class 1A PI(3)Ks, does not disturb localization of PHAKT-EGFP at the leading edge, suggesting that class 1A PI(3)Ks are not involved in PI(3,4,5)P3-PI(3,4)P2 gradient formation. (C)PI(3,4,5)P3-PI(3,4)P2 pulse at the tail upon turning. Time-lapse ratiometric imaging of PHAKT-EGFP/mCherry at low magnification during locomotion of neutrophils in the mesenchymal tissues of the head (Movie S4B). PI(3,4,5)P3-PI(3,4)P2 pulse appears at the tail (magenta arrowhead) upon turning or changing directions in addition to the leading edge (green arrowhead). White arrows indicate direction of migration. Note that blue neutrophils have balanced expression of both PHAKT-EGFP and mCherry but that multi-colored neutrophils have high expression of PHAKT-EGFP with low or no expression of mCherry. Scale bars, 10 μm. |
|
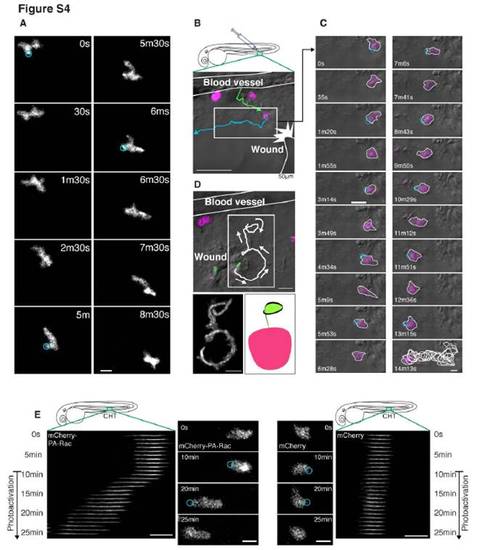
Photoactivation of PA-Rac (related to Figure 4) (A) Laser stimulation with 458nm light does not have any obvious effects on morphology or migration of neutrophils expressing only mCherry in Tg(MPO:mCherry)uw. The circles indicate positions of laser stimulation. (B) (C) Photoactivation of PA-Rac disturbs attraction of a neutrophil to a wound in the fin of Tg(MPO:mCherry-PA-Rac1)uw. The green and blue lines are tracking of the neutrophil before and after photoactivation. The circles indicate positions of laser stimulation. (D) Photoactivation of PA-Rac enables guiding of a neutrophil between a wound and the blood vessel, indicating localized Rac activation can overcome endogenous chemotaxis signaling. The trajectory of the neutrophil draws an “apple”. (E) Photoactivation of PA-Rac is sufficient to induce directed migration of relatively immotile neutrophils in the CHT. Laser stimulation with 458nm light induces migration of a neutrophil with mCherry-PA-Rac (left), but not with mCherry (right). Repetitive photoactivation was performed 10 minutes after starting the imaging (the circles indicate position of photoactivation). To make the kymograph, Z-stack images were summed into a single two-dimensional image, and then consolidated into a semi-one-dimensional line. Scale bars, 10 μm (A, C, E), 50 μm (B), 20 μm (D). |
|
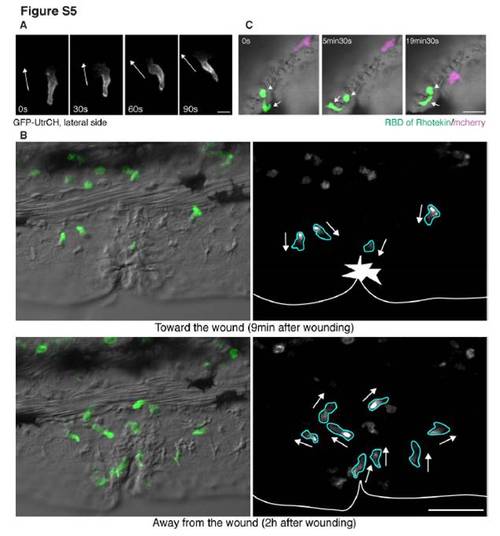
Imaging uropod events (related to Figure 5) (A) GFP-UtrCH (a biosensor for stable F-actin) labels the lateral sides of a neutrophil. Stable F-actin is localized at the lateral sides in addition to the tail during neutrophil random migration in the mesenchymal tissues of the head. The white arrows indicate direction of migration. (B) GFP-UtrCH (a biosensor for stable F-actin) labels the tail and lateral sides of neutrophils when they migrate towards or away from a wound in the fin of Tg(MPO:EGFP-UtrCH)uw (movie S10). (C) Inhibition of RhoA makes the tail round. EGFP-rGBD (RhoA binding domain of rhotekin fused to EGFP) was overexpressed into Tg(MPO:mCherry)uw to inhibit RhoA activity (Worthylake et al., 2001). Inhibition of RhoA activity disturbs cell migration in vivo and induces a rounded morphology of the tail (movie S12B). The arrow indicates the leading edge, which appears normal, while the arrowhead shows the round tail. Note that the magenta neutrophil expressing only mCherry migrates normally. See also movie S12C, in which RhoA is disturbed by overexpression of Rho T19N. Scale bars, 10 μm (A), 50 μm (B), 20μm (C). |
|
Transient activation of Rac with PI3K inhibition induces accumulation of stable F-actin at the protrusion, which contracts and pushes the cell body to the opposite direction (related to Figure 6). Rac was photoactivated once at the edge of a cell treated with the PI(3)Kγ-specific inhibitor AS-605240 (movie S13C). After stopping photoactivation, stable F-actin accumulates at the protrusion induced by Rac activation, which contracts and pushes the cell body to the opposite direction. The white arrows indicate direction of cell movement. Scale bar, 10 μm. |
Reprinted from Developmental Cell, 18(2), Yoo, S.K., Deng, Q., Cavnar, P.J., Wu, Y.I., Hahn, K.M., and Huttenlocher, A., Differential Regulation of Protrusion and Polarity by PI(3)K during Neutrophil Motility in Live Zebrafish, 226-236, Copyright (2010) with permission from Elsevier. Full text @ Dev. Cell