- Title
-
Induction and patterning of trunk and tail neural ectoderm by the homeobox gene eve1 in zebrafish embryos
- Authors
- Cruz, C., Maegawa, S., Weinberg, E.S., Wilson, S.W., Dawid, I.B., and Kudoh, T.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
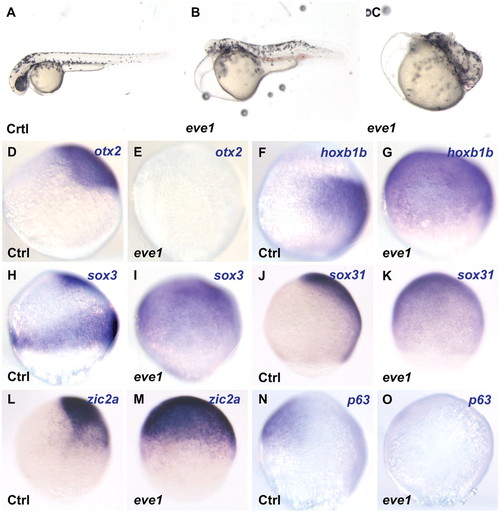
Eve1 overexpression causes anterior truncation, suppression of anterior markers, and induction of posterior markers. Zebrafish embryos were injected with eve1 mRNA (as indicated at the bottom left corner of each panel; Ctrl, uninjected controls). (A–C) Eve1 mRNA injected embryos at 48 hpf showing anterior truncation and progressive loss of posterior patterning. (D–O) In situ hybridization of control and eve1 mRNA-injected embryos at 80% epiboly (lateral views, dorsal to the right, where discernible). Genes analyzed are indicated in the top right corner. Expression of the anterior gene otx2 and epidermal gene p63 was suppressed (D, E; N, O), whereas the expression of hoxb1b was expanded by eve1 overexpression (F, G). Expansion also was observed for sox3, zic2a, and sox31 (H–M). EXPRESSION / LABELING:
|
|
Eve1 depletion suppresses trunk and tail development, and eve1 acts as a repressor. Zebrafish embryos were injected with eve1MO (B, C, H, L, P, T) or with eve1-VP16 mRNA (E, F, I, M, Q, U) as shown at bottom right of panels B, C, E, and F and at the top of the columns for the remainder. Embryos at 24 hpf (A–C) and at 28 hpf (D–F), show variable loss of trunk and tail tissue. (G–V) In situ staining of embryos at 70–80% epiboly (G–R) and 60% epiboly (S–V): lateral views, dorsal to the right (where discernible), with probes shown at the left of the rows. Hoxb1b and aldh1a2 are suppressed by eve1MO (H and L) and eve1-VP16 (I and M, compare with G and K), and expression of both genes is expanded in embryos injected with eve1-Eng (J and N). Conversely, bmp2b and bmp4 expression domains are expanded in embryos injected with eve1MO (P and T) and eve1-VP16 (Q and U, compare with O and S), whereas eve1-Eng suppresses expression of both BMPs (R and V). EXPRESSION / LABELING:
|
|
Eve1 induces hoxb1b expression via an RA signal. (A–J and M–S) Dorsal and (K and L) lateral views (where discernible, dorsal to the right) of zebrafish embryos fixed for in situ staining at 80% epiboly (A–L) and 60% epiboly (M–S) Injections are indicated at the bottom left of each panel, and genes analyzed are given at the top right. (A–D) Suppression of otx2 by eve1 does not depend on RA because it resists overexpression of the RA-metabolizing enzyme Cyp26a1. (E–H) Eve1-mediated induction of hoxb1b does not occur when eve1 and cyp26a1 mRNAs are coinjected (G), and cyp26a1 injection alone suppresses hoxb1b expression (H; only one of two cells was injected in this embryo). (I and J) Eve1 induces aldh1a2 expression. (K and L) Anterior expression of cyp26a1 is suppressed by eve1 but remains unaffected at the margin. (M–O) Injection of high concentrations of aldMO and eve1 mRNA (Materials and Methods). Eve1 cannot rescue hoxb1b expression in aldMO-injected embryos (O). (P–S) Injection of low concentrations of eve1MO (2 ng/nL) and aldMO showed synergism in the suppression of hoxb1b. EXPRESSION / LABELING:
|
|
Interactions between eve1 and BMP. Lateral views (where discernible, dorsal to the right) of zebrafish embryos at 70–80% epiboly (A–S). Embryos were injected at the one-cell stage (A–K, M, and Q) or at the one– to four-cell stage (N and R). For coinjection of eve1 mRNA and bmp2bMO, embryos first were injected with eve1 at the one-cell stage and then were injected with bmp2bMO at the four- to eight-cell stage (O and S). Genes analyzed are indicated at the left of the rows; injections are indicated at the top of columns. Bmp2b suppresses neural markers sox3 and hoxb1b even in the presence of eve1 (A–F). Bmp4 expression is suppressed by eve1 (H) but is ubiquitously induced by coinjection of bmp2b mRNA (I, compare to G). Bmp2b expression also is suppressed by eve1 (J, K). Eve1 and bmp2bMO synergize in ectodermal fate specification (L–S). Low levels of eve1 mRNA (10 pg/nL) or bmp2bMO (100 pg/nL) injected individually do not affect sox3 or foxi.1 expression (M, N, Q, and R), but coinjection at the same concentrations induced sox3 (O) and suppressed foxi.1 (S). EXPRESSION / LABELING:
|
|
Eve1 rescues posterior neural development in ich-/- mutants. Homozygous ich-/- embryos were injected with eve1 mRNA. Genotype is indicated at the bottom left of each panel, injections at the bottom right, and in situ probes at the top right. Uninjected ich-/- embryos at 24 hpf (A). Eve1 mRNA injection leads to varying levels of rescue of posterior dorsal axis (C–E). Embryos stained for hoxb1b at 80% epiboly, presumed lateral view, dorsal to the right. Expression of hoxb1b is absent in uninjected ich-/- embryos (C, D) but is rescued by injection of eve1 mRNA (E). In situ hybridization of wild-type and ich-/- embryos at 24 hpf (anterior to the left) (F-Q). Neural gene expression and posterior dorsal axis formation was partially rescued by the injection of eve1 mRNA. Rescue of pax2a appears to extend to the midbrain–hindbrain boundary (L-N, arrowheads), whereas egr2b expression appears to extend to rhombomere 5 (O–Q, arrowheads). EXPRESSION / LABELING:
PHENOTYPE:
|
|
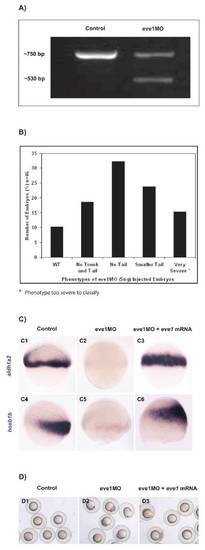
Eve1MO phenotypic classes and specificity of eve1MO. (A) PCR of eve1 transcripts in uninjected and eve1MO-injected embryos. Note the two bands in the eve1MO lane compared with the single band in control embryos, which corresponds to the correct size for eve1 mature mRNA. Sizes are indicated to the left. (B) Range of phenotypes observed in eve1MO-injected embryos. (C) Eve1 mRNA injection rescues the eve1MO-mediated suppression of aldh1a2 (C3) and of hoxb1b (C6), indicating that the MO is specific. (D) Eve1 mRNA injection rescues eve1MO-induced epiboly defects; photographs were taken when control embryos reached 80% epiboly. |
|
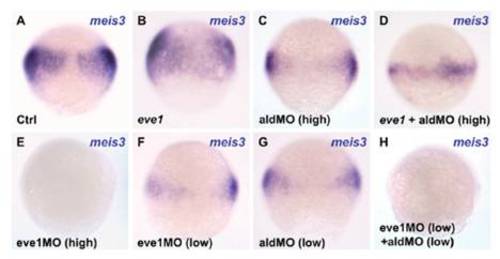
Eve1 induces meis3 expression via an RA signal. Dorsal views (where discernible) of zebrafish embryos fixed for in situ staining at 70% epiboly. Injections are indicated at the bottom left of each panel (Ctrl, control), and the genes analyzed are indicated at the top right. (A–D) Injection of eve1 mRNA and high concentrations of aldMO (Materials and Methods). Eve1 cannot rescue meis3 expression in aldMO-injected embryos (D). High levels of eve1MO (5 ng/ nL; Materials and Methods) suppresses meis3 expression (E). (F–H) Injection of low concentrations of eve1MO (2 ng/nL) and aldMO indicates synergism between eve1 and aldh1a2 in the regulation of meis3. |
|
Eve1 regulates BMP expression but not BMP signaling. Lateral views (where discernible, dorsal to the right) of zebrafish embryos fixed for in situ staining at 80% epiboly. Genes analyzed are indicated at the left of the rows, and injections are indicated at the top of the columns. Induction of the neural markers sox3 (B) and hoxb1b (F) and suppression of bmp4 (J) by eve1 is antagonized even by low doses (7 pg/nL) of bmp2b coinjected with eve1 [100%; n = 25 (C), 100%, n = 34 (G), and 100%, n = 21 (K) respectively] (compare to A, E, and I). (D, H, and L) Expression of sox3 (D), hoxb1b (H), or bmp4 (L) following injection of low doses (7 pg/nL) of bmp2b. |