- Title
-
Pivotal role of hmx2 and hmx3 in zebrafish inner ear and lateral line development
- Authors
- Feng, Y., and Xu, Q.
- Source
- Full text @ Dev. Biol.
|
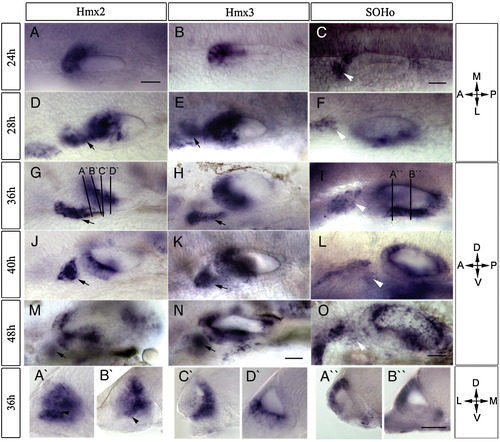
Expression of hmx genes during development of the zebrafish otic vesicle. RNA transcripts were detected using whole mount in situ hybridization. Probes, stages, and orientation are indicated, with the plane of transverse sections (A′–D′ and A″, B″) shown in panels G and I. Both hmx2 (A, D, G, J, M) and hmx3 (B, E, H, K, N) are expressed in the anterior otic vesicle and in the cells anterior to the otic vesicle (arrows). SOHo (C, F, I, L, O) is expressed in the postero-ventral part of the otic vesicle and the anterior lateral line ganglion (white arrowheads). Expression of all three hmx genes is restricted to the area of semicircular canals at later stages. Scale bars: (A–O) 50 μm, (A′–D′, A″, B″) 25 μm. Abbreviations: A, anterior; P, posterior; M, medial; L, lateral; D, dorsal; V, ventral. |
|
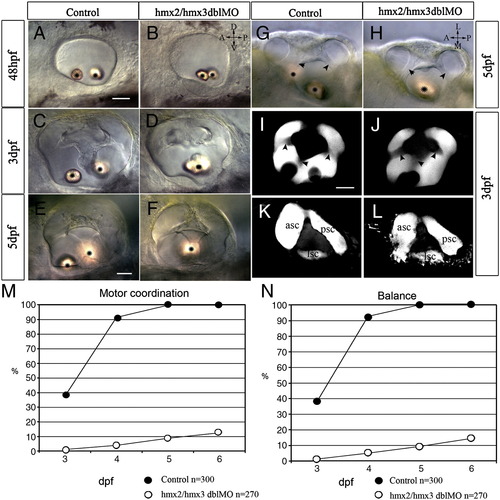
Ear morphology and vestibular function defects in hmx2/hmx3 morphant embryos. All images are anterior to the left. Bright field DIC images showing normal overall morphology of the ear with fused otoliths (asterisk) in hmx2/hmx3 morphants at the stages indicated (A–H). Lateral view in panels A–F, I–L; dorsal view in panels G, H. Normal semicircular canals in control embryos (I, K) and morphants (J, L) revealed by confocal Z-projection of dye filled lumen. Superficial focal plane in panels I, J, and deeper plane in panels K, L. Arrowheads indicate the lumen of the semicircular canal. Scale bar: 25 μm. Vestibular function is expressed as motor coordination (M) and balance (N) tested for the control embryos and morphants. The x-axis is time (dpf) and the y-axis is percentage of embryos scored positive for the test. Sample size (n = ) is indicated. Abbreviations: ASC, anterior semicircular canal; LSC, lateral semicircular canal; PSC, posterior semicircular canal. |
|
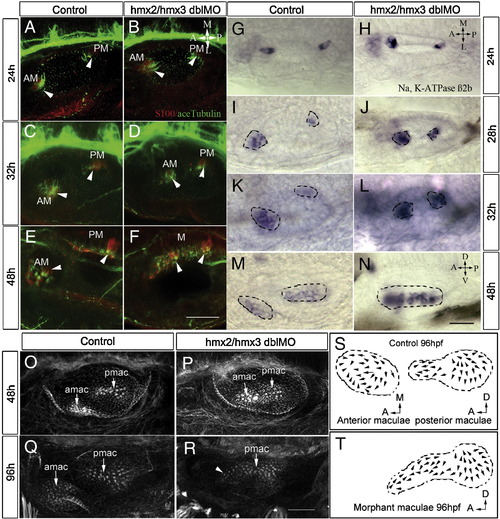
Reduced number of hair cells and progressive fusion of two maculae in hmx2/hmx3 morphants. Anterior to the left, dorsal view in panels A–L and lateral view in panels M–R. (A–F) Hair cells are labeled with antibodies for S-100 (red) and acetylated-tubulin (green) at the stages indicated showing progressive fusion of the anterior (utricular) maculae with the posterior (saccular) maculae at the medial wall in the morphants. (G–N) In situ hybridization with Na,K-ATPase β2b probe to reveal mature hair cells, showing a reduced number in the anterior maculae (amac) and fusion with the posterior maculae (pmac). (O–R) Rhodamine–phalloidin staining of the F-actin of the hair bundles in control (O, Q) and morphants (P, R). (S) Illustration of control amac and pmac from 9 ears. (T) Illustration of fused maculae in morphants from 8 ears. Arrowheads indicate hair cells. Scale bars: (A–F) 50 μm, (G–R) 25 μm. Abbreviations: AM, anterior maculae; PM, posterior maculae; M, maculae fusion. |
|
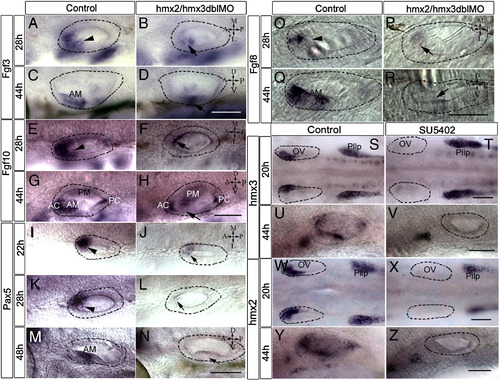
Expression of fgf3, fgf8, fgf10, and pax5 in hmx2/hmx3 morphants and of hmx2 and hmx3 in SU5402-treated embryos. All images are anterior to the left, and orientations are indicated. Dorsal view at 28 hpf and lateral view at 44 hpf. Otic vesicle is highlighted with a dotted line. fgf3 ( A, C) control, (B, D) morphant; fgf10 (E, G) control, (F, H) morphants; fgf8 ( O, Q) control, ( P, R) morphants; pax5 (I, K, M) control, (J, L, N) morphants. Dorsal view in panels S–Z. Embryos treated in 5% DMSO with or without SU5402 from 14 to 21 hpf (S, T, W, X) or 24 s to 44 hpf (U, V, Y, Z). hmx3 (S–V); hmx2 (W–Z). Arrowhead indicates the normal expression, and arrow indicates reduced signals. Scale bar: 50 μm. Abbreviations: OV, otic vesicle; Pllp, posterior lateral line primordium; AM, anterior maculae; PM, posterior maculae; AC, anterior cristae; PC, posterior cristae. EXPRESSION / LABELING:
|
|
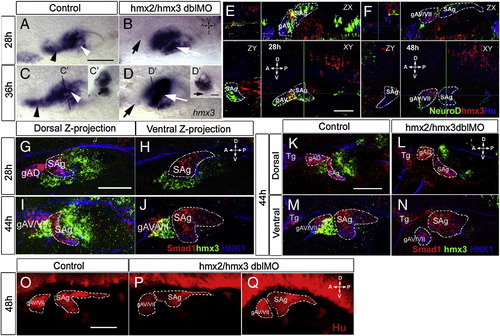
Analysis of sensory ganglion formation in hmx2/hmx3 morphant embryos. Anterior is to the left and the orientation as indicated. (A–D) hmx3 expression in control (A, C) and morphant (B, D) embryos, with positions of the transverse sections (C′, D′) as indicated. The black arrowhead indicates hmx3-expressing cells anterior to the otic vesicle in the control, and the black arrow indicates the absence of this cell population in morphants. The white arrowhead indicates the ventral floor in control embryos and the white arrow indicates hmx3-expressing cells retained at the ventral floor in morphants. (E, F) Confocal images of a single stack, showing expression of hmx3 (red), neuroD (green) and anti-Hu (blue) in the otic vesicle of wild-type embryos at 28 hpf (E) and 48 hpf (F). The green and blue lines in XY plane indicate the position of the ZX and ZY sections, respectively. hmx3-expressing cells (red) are only present in gAV at 28 hpf and gAV/VII at 48 hpf. (G–N) Triple detection of smad1 (red), hmx3 (green), and anti-HNK1 (blue) in wild-type (G–K, M) or hmx2/3 morphant (L, N) embryos at the indicated stages. The pictures show projections of confocal stacks through either the dorsal (G, I, K, L) or ventral (H, J, M, N) part of the otic vesicle. (O–Q) Detection of Hu protein in control (O) and hmx2/3 morphant (P, Q) embryos. Dotted lines outline the structure indicated in the panel. Abbreviations: gAD, dorsal branch of the anterior lateral line ganglion; gAV/VII, ventral branch of the anterior lateral line ganglion (gAV) fused with facial sensory ganglion (gVII); SAg, statoacoustic ganglion; Tg, trigeminal ganglion. Scale bar: 50 μm except 25 μm in panel D′. |
|
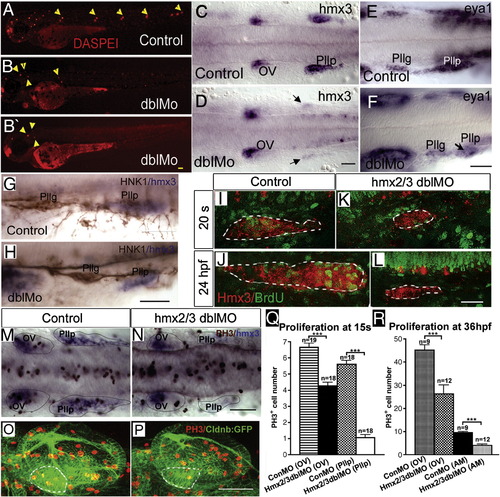
Lateral line development and cell proliferation in hmx2/hmx3 morphant embryos. All images are anterior to the left in lateral view (A, B, B′, G, H, I–L, O, P) or dorsal view (C–F, M, N). DASPEI staining of 3dpf embryos in control (A) and morphant embryos (B, B′) showing reduced number of neuromasts (yellow arrowheads) in hmx2/hmx3 morphants; (C) control and (D) morphant expression of hmx3 at 24 hpf, (E) control and (F) morphant expression of eya1 at 24 hpf; (G) control and (H) morphant embryos stained with anti-HNK1 (brown) and hmx3 (blue); (I–L) BrdU incorporation, (I, J) control and (K, L) morphant posterior lateral line primordium labeled with hmx3 (red) and BrdU (green); (M, N) double labeling of hmx3 (blue) with anti-PH3 (brown) at 15 somites in control (M) and morphants (N), showing reduced number of anti-PH3-positive cells in the hmx3 expression domain (highlighted with dotted line); (O, P) anti-PH3 (red) labeling of proliferating cells in the otic vesicle (OV) of control (O) and morphant embryo (P), in CldnB:lynGFP transgenic line. Dotted line outlines the anterior maculae (AM) region in both panels. Scale bar: 50 μm. (Q) Quantification of PH3-positive cells in hmx3 expression domain represented in panels M and N. Numbers of PH3-positive cells are 6.67 ± 1.20 in control OV; 4.28 ± 0.88 in hmx2/hmx3 morphant OV; 5.6 ± 1.0 in control Pllp and 1.0 ± 0.82 in morphant Pllp at 15 s. (R) Quantification of PH3-positive cells in the OV and AM, respectively, in control and morphant embryos. The number of proliferating cells was counted from the confocal stacks and quantified. Number of PH3-positive cells are 45.2 ± 2.18 in control OV, 26.2 ± 3.87 in morphant OV; 9.56 ± 0.41 in control AM, 4.08 ± 0.53 in morphant AM. Sample size (n = ) is indicated. Student test (t) is used to compare the control and morphant samples in Excel and error bars represent the standard deviation in each sample set. Abbreviation: AM, anterior maculae; OV, otic vesicle; s, somite. EXPRESSION / LABELING:
PHENOTYPE:
|
|
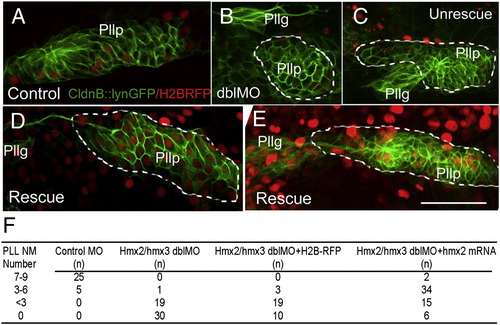
Mosaic expression of Hmx partially rescues the phenotype in posterior lateral line of hmx2/hmx3 morphants. Anterior to the left. The posterior lateral line primordium is labeled with the membrane GFP in CldnB::lynGFP transgenic embryo. (A) Normal control primordium injected with H2BRFP RNA (red) at 24 hpf. (B–E) Rescue experiment with co-injection of Hmx2 and H2BRFP RNAs (red) in hmx2/hmx3 morphant embryos. (B, C) Abnormal primordium of morphants without Hmx2 and H2BRFP injection. (D, E) Rescued primordium following Hmx2 and H2BRFP injection in morphants. (F) Summary of the number of neuromasts formed in each injection group. Embryos were stained for the endogenous alkaline phosphatase activity at 3dpf and number of neuromasts in each embryo counted. Sample size (n) is indicated. Scale bar: 50 μm. Abbreviations: Pllp, posterior lateral line primordium; PLL NM, posterior lateral line neuromast. EXPRESSION / LABELING:
|
|
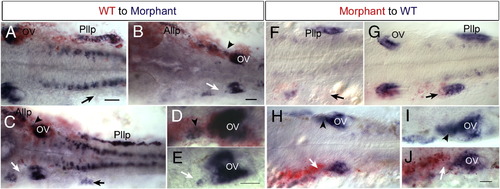
Cell autonomous effect of Hmx2 and Hmx3 function. Anterior to the left and dorsal view. Donor cells are labeled in red and hmx3 expression in blue. (A–E) Examples of wild-type cells transplanted into hmx2/hmx3 morphant embryos. Arrowheads indicate wild-type cells in morphant backgrounds. Arrows indicated affected tissue in morphant embryos. (F–J) Morphant cells transplanted into wild-type embryos. Arrows indicate morphant donor cells in defective lateral line primordium or otic vesicle. Arrowheads indicate normal development in the control side. Scale bar: 50 μm. Abbreviations: OV, otic vesicle; Pllg, posterior lateral line ganglion; Pllp, posterior lateral primordium; WT, wild type. |
|
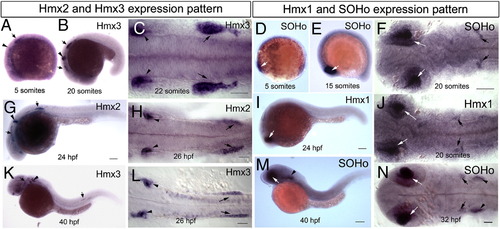
Developmental expression of hmx genes in zebrafish embryos. Whole mount in situ hybridization with antisense probles of hmx3 ( A–C, K, L), hmx2 (G, H), SOHo ( D–F, M, N), and hmx1 (I, J) at stages indicated. Arrows indicate anterior and posterior lateral line ganglion, arrowheads indicate otic vesicle and white arrows indicate eye. Scale bar: 25 μm. EXPRESSION / LABELING:
|
|
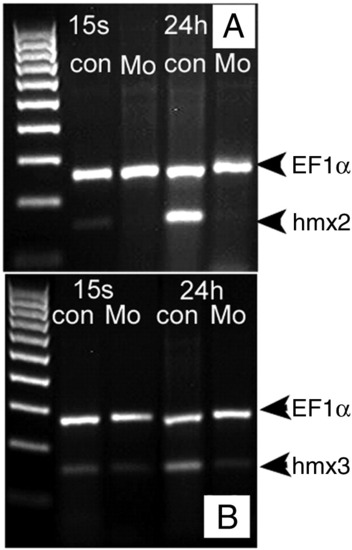
RT-PCR verification of the efficiency by splice blocking morpholino. Samples were collected at 15 somites (16.5 hpf) and 24 hpf, respectively. ef1α is used as internal control. A, hmx2 splice blocker morphant sample. B, hmx3 splice blocker morphant sample. |

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 339(2), Feng, Y., and Xu, Q., Pivotal role of hmx2 and hmx3 in zebrafish inner ear and lateral line development, 507-518, Copyright (2010) with permission from Elsevier. Full text @ Dev. Biol.