- Title
-
Dnmt3 and G9a cooperate for tissue-specific development in zebrafish
- Authors
- Rai, K., Jafri, I.F., Chidester, S., James, S.R., Karpf, A.R., Cairns, B.R., and Jones, D.A.
- Source
- Full text @ J. Biol. Chem.
|
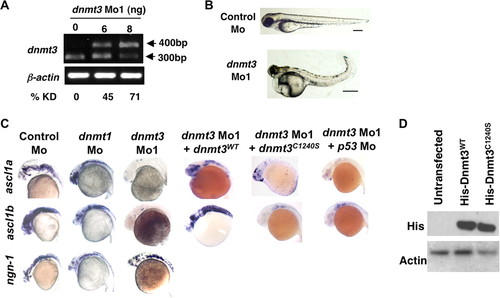
Dnmt3 knockdown in zebrafish embryos results in neurogenesis defects. A, splice blocking by dnmt3 splice-blocking morpholino (dnmt3 Mo1) was monitored by RT-PCR in mRNAs from control and dnmt3 morphants at 80 hpf. PCR was performed using a forward primer in exon 6 and a reverse primer in exon 7. Note that the dnmt3 Mo1 stabilizes the unspliced transcript containing introns 6 and 7 (400 bp), whereas the spliced version (300 bp) is detected in control injected embryos. Percent knockdown (% KD) shown is the ratio of intensity of unspliced product to combined intensity of unspliced and spliced products normalized over β-actin intensity. B, morphology defects in dnmt3 morphants at 80 hpf. Note the smaller head, a pericardial edema, and a curled tail in dnmt3 morphants compared with control morphants. Bar equals 0.5 mm. C, whole mount in situ analysis of ngn-1, ascl1a, and ascl1b expression in dnmt3 morpholino alone or with p53 morpholino-, dnmt1 morpholino-, or control morpholino-injected embryos at 30 hpf. Whereas ngn-1 expression was normal in dnmt3 and dnmt1 morphants, ascl1a and ascl1b were selectively is absent. This defect can be complemented by co-injection of the wild-type (Dnmt3WT) but not by a catalytically inactive derivative (Dnmt3C1240S). D, protein expression levels of Dnmt3 wild-type (Dnmt3WT) and catalytically inactive (Dnmt3C1240S) derivatives. HEK293 cells were transfected with Dnmt3WT and Dnmt3C1240S, and Western blots were performed using antibodies against His tag. Both of these show equal expression of these derivatives. β-Actin was used as a loading control. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Distinct tissue-specific developmental defects in dnmt1 and dnmt3 morphants. A, C, and E, whole mount in situ analysis of dhand (at 48 hpf) (A), fabp2, trypsin, fabp10, insulin, and trypsin (C), and irbp (E) (all at 96 hpf) in dnmt1, dnmt3, and control morphants. In panel A, arrows show loss of last two pharyngeal pouches in dnmt3 morphants. In panel C, arrows point to expression of gata6 in the pancreas. B, Alcian Blue staining of Control, dnmt1, or dnmt3 morphants at 96 hpf shows loss of jaw structure in dnmt1 morphants but not in dnmt3 morphants. D, toluidine blue staining of histological cross-sections within the dnmt3 morphant retinas at 80 hpf. Note the loss of organization of different retinal layers in dnmt3 morphants. F, quantitative RT-PCR for Opsin Shortwave 1 and Opsin Shortwave 2 in control, dnmt1, or dnmt3 morpholino-injected embryos at 96 hpf. |
|
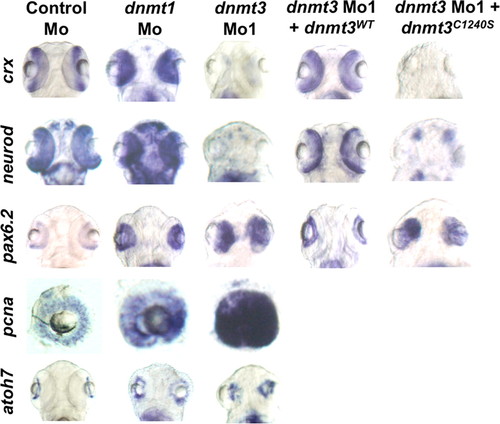
Dnmt3 morphants are defective in retinal differentiation. Whole mount in situ for pax6.2, crx, and neurod in dnmt3 and control morphants at 80 hpf. Whereas all the retinal cells expressed pax6.2, a marker of retinal progenitors, markers of specific progenitors (crx and neurod) were absent in dnmt3 morphants. These defects can be complemented by co-injection of the wild-type Dnmt3 (Dnmt3WT) but not with the catalytically inactive Dnmt3 derivative (Dnmt3C1240S). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Dnmt3 overexpression in dnmt1 morphants and Dnmt1 overexpression in dnmt3 morphants does not rescue developmental defects. A and B, whole mount in situ expression for ascl1a, ascl1b, crx, and neurod (A) and fabp2 and irbp (B) in embryos injected with control morpholino, dnmt3 morpholino alone, or with DNMT1 mRNA (A) and dnmt1 morpholino alone or with dnmt3 mRNA (B). EXPRESSION / LABELING:
|
|
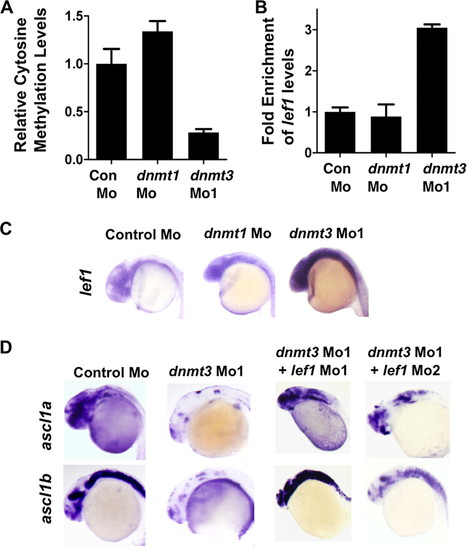
Lef1 repression by Dnmt3 is critical for proper neurogenesis. A, MeDIP-PCR based quantification of methylation status of lef1 promoter in control, dnmt1, and dnmt3 morphant embryos at 24 hpf. The y-axis represents values at lef1 promoter first normalized to a negative region (with an insignificant number of CpGs in 1000 bp vicinity) and then normalized to control Mo values as 1. B, graph showing quantitative RT-PCR results for zebrafish lef1 normalized to 28 S values in control morpholino-, dnmt1 morpholino-, or dnmt3 morpholino-injected embryos at 24 hpf. Results are represented in a -fold change format where the lef1/28S ratio from control morphants was normalized to 1. C, expression of lef1 in control, dnmt1, and dnmt3 morphant embryos at 24 hpf as detected by whole mount in situ hybridization. D, whole mount in situ analysis of ascl1a and ascl1b expression in embryos (30 hpf) injected with control morpholino and dnmt3 morpholino co-injected with either lef1 Mo1 or lef1 Mo2. |
|
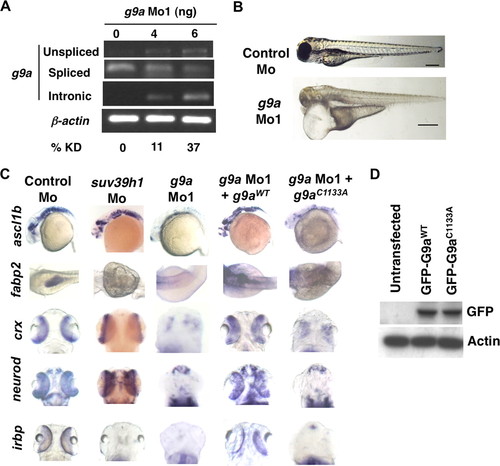
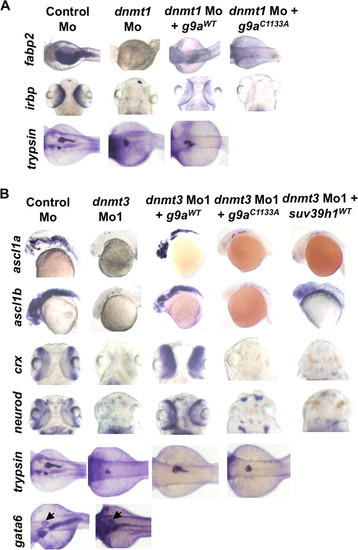
G9a morphants largely phenocopy dnmt3 morphants. A, splice blocking by g9a splice-blocking morpholino (g9a Mo1) was monitored by RT-PCR in mRNAs made from control and g9a morphants at 80 hpf using a forward primer in the exon and reverse primer in the next exon. Note that the g9a morpholino stabilizes the unspliced transcript containing the intermediate intron, whereas the spliced version is detected in control-injected embryos. The percent knockdown shown is the ratio of intensity of unspliced product to combined intensity of unspliced and spliced products normalized to intensity of the β-actin band. Note that a product can be amplified with an intronic primer in the g9a morphants; however, this was not taken into account while calculating percent knockdown. B, morphology defects in g9a morphants at 80 hpf. Note the smaller head in g9a morphants, as with dnmt3 morphants. Bar equals 0.5 mm. C, whole mount in situ analysis of ascl1b, crx, neurod, fabp2, and irbp expression in g9a, suv39h1, and control morphants. ascl1b was analyzed at 30 hpf, whereas all others were analyzed at 80 hpf. The defects present in g9a morphants were complemented by co-injection of the wild-type G9a (G9aWT), but not by the catalytically inactive derivative G9aC1133A. D, expression of exogenous G9a constructs. HEK293 cells were transfected with the wild-type or catalytically dead derivatives of GFP-tagged zebrafish G9a (G9aWT and G9aC1133A). Westerns using antibodies against the GFP tag show equal expression of these derivatives. β-Actin was used a loading control. EXPRESSION / LABELING:
PHENOTYPE:
|
|
G9a overexpression rescues both dnmt3 morphants and dnmt1 morphants. Whole mount in situ analysis of ascl1a, ascl1b, crx, and neurod (A) and fabp2, irbp, and trypsin (B) expression in embryos injected with control morpholino or dnmt3 or dnmt1 morpholino co-injected with wild-type or catalytically null G9a or Suv39h1. ascl1a and ascl1b were analyzed at 30 hpf, whereas all others were analyzed at 80 hpf. Note that co-injection of wild-type G9a (G9aWT) but not of either catalytically inactive G9a (G9aC1133S) or wild-type Suv39h1 rescues the ascl1a and ascl1b expression in dnmt3 morphants. Overexpression of wild-type G9a, but not catalytically inactive G9a, can also rescue fabp2 and irbp expression in dnmt1 morphants. Of note, wild-type G9a could not rescue trypsin expression in dnmt1 morphants. The panel showing Suv39h1 rescue of dnmt1 morphants has been reported previously (7) and is shown for comparative purposes. EXPRESSION / LABELING:
|
|
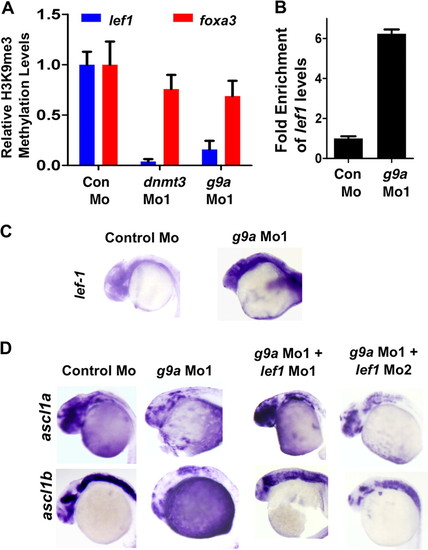
Lef1 repression by G9a is critical for proper neurogenesis. A, quantitative PCR for chromatin immunoprecipitation for H3K9me3 marks on lef1 promoter (Blue) or on foxa3 promoter (Red) in control morphant, dnmt3 morphant, and g9a morphant embryos. Values shown represent enrichment on the experimental region normalized to values on control region. B, graph showing quantitative RT-PCR results for zebrafish lef1 normalized to 28S values in control morpholino or g9a morpholino-injected embryos at 24 hpf. Results are represented in a -fold change format where the lef1/28S ratio from control morphants was normalized to 1. C, expression of lef1 in control and g9a morphant embryos at 24 hpf as detected by whole mount in situ hybridization. D, whole mount in situ analysis of ascl1a and ascl1b expression in embryos (30 hpf) injected with control morpholino and g9a morpholino co-injected with either lef1 Mo1 or lef1 Mo2. |
|
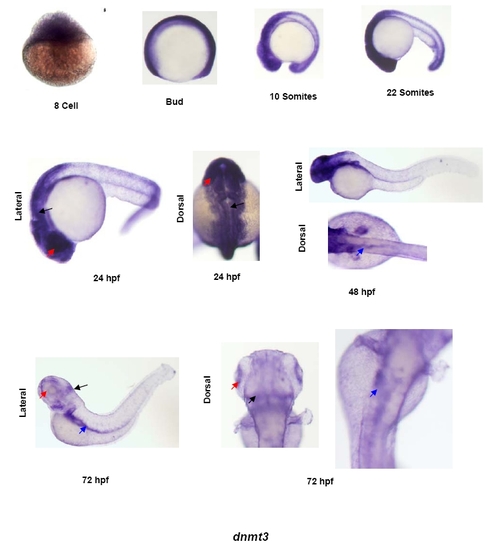
Expression pattern of dnmt3 in developing zebrafish embryos. Whole mount in situ analysis of dnmt3 expression in different stages of zebrafish development. Shown here are 8-cell, bud, 10-somites, 22-somites, 24hpf, 48hpf and 72hpf stages. For first four stages only lateral views are shown whereas for last three time points dorsal and lateral images are shown to depict tissue-specific expression. Black arrows show expression in brain, red arrows denote expression in the eye and blue arrows point to the expression in the gut. |
|
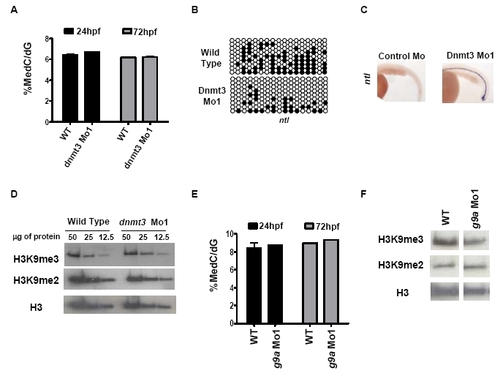
G9a and dnmt3 morphants have wild type 5-methyl-deoxycytosine and histone H3K9 di- or tri-methyl levels. (A) LC-MS assay was performed on genomic DNA isolated from dnmt3 morphants and wild type embryos at 24hpf and 72hpf. Note that no change in global 5-methyl-deoxycytosine levels was detected in dnmt3 morphants compared to wild type embryos at the time points tested. (B) Schematic presentation of bisulfite sequencing data for ntl CpG island in wild type and dnmt3 morphants at 24hpf. Black and white circles represent methylated and unmethylated cytosines in CpG sequences, respectively. (C) Expression of ntl (ventral view) in control and dnmt3 morphant embryos at 24hpf as detected by whole mount in situ hybridization. (D) Protein was isolated from wild type and dnmt3 morphants at 80hpf and H3K9me2 and H3K9me3 levels were measured by antibodies specific to these marks. Decreasing amounts (as shown in microgram quantities) of the proteins were loaded for proteins from dnmt3 morphants or wild type embryos. (E) LC-MS assay was performed on genomic DNA isolated from g9a morphants and wild type embryos at 24hpf and 72hpf. Note that no change in global 5-methyl-deoxycytosine levels was detected in g9a morphants compared to wild type embryos at the time points tested. (F) Protein was isolated from wild type and g9a morphants at 80hpf and H3K9me2 and H3K9me3 levels were measured by antibodies specific to these marks. Twenty five micrograms of total protein isolated from g9a morphants or wild type embryos was loaded in each lane. No detectable change was observed in global levels of H3K9me2 or H3K9me3 in dnmt3 or g9a morphants when compared to wild type embryos. Pan-H3 C-terminal antibody was used for total H3 content. EXPRESSION / LABELING:
|
|
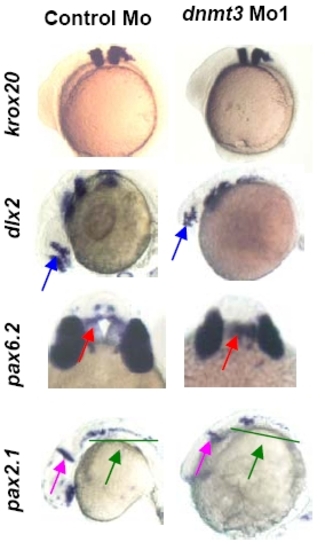
Dnmt3 morphants are not defective in the patterning of brain. Whole mount in situ of krox20 (at 3 somites), dlx2, pax6.2, and pax2.1 (all three at 24hpf) expression in Dnmt3 and control morphants. Krox20 (rhombomeres 3 and 5), dlx2 (diencephalons and telencephalon, blue arrow), pax6.2 (dorsal diencephalons, red arrow), and pax2.1dnmt3 morphants. |
|
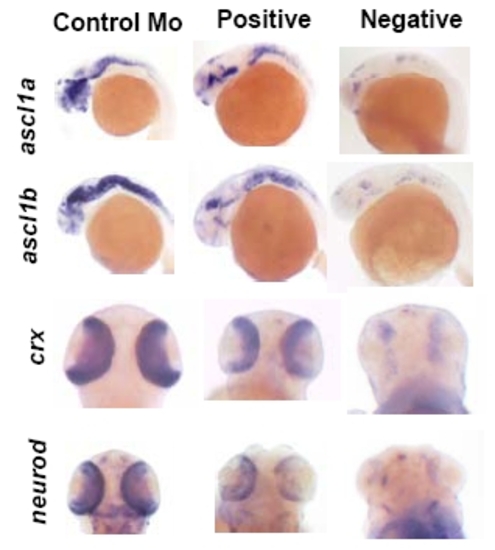
Examples of positive and negative staining. Examples of embryos counted as positive or negative is shown for ascl1a, ascl1b, crx and neurod. |
|
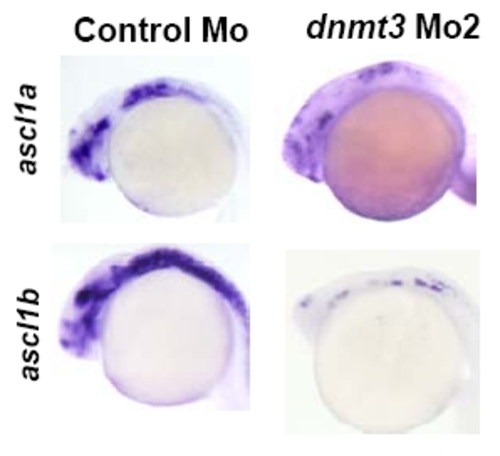
Dnmt3 morphants are defective in brain neurogenesis. Whole mount in situ analysis of, ascl1a and ascl1b expression in a second dnmt3 morpholino (translation blocker, 8ng) or control morpholino injected embryos at 30hpf. |
|
Dnmt3 morphants show abnormal brain structure. (A) Bright light image of dnmt3 and control morphants at 24hpf shows that brain structure was not formed correctly. Note that Control morphants are transparent and internal structures can be seen whereas dnmt3 morphants are filled with black spots which depict cells undergoing apoptosis or necrosis. Embryos co-injected with dnmt3 morpholino and p53 morpholino together show reduced cell death. (B) p53 morpholino knockdown. PCR was performed on cDNA made from embryos injected with control morpholino or p53 morpholino and dnmt3 morpholino at 24hpf. Unspliced and spliced DNA length for p53 is shown by arrows. |
|
Expression pattern of g9a in developing zebrafish embryos. Whole mount in situ analysis of g9a expression in different stages of zebrafish development. Shown here are 1-cell, bud, 10-somites, 24hpf, 48hpf and 72hpf stages. For first three stages only lateral views are shown whereas for last three time points dorsal and lateral images are shown to depict tissue-specific expression. Black arrows show expression in brain, red arrows denote expression in the eye and blue arrows point to the expression in the gut. EXPRESSION / LABELING:
|
|
G9a morphants are defective in brain neurogenesis. Whole mount in situ analysis of, ascl1a and ascl1b expression in a second g9a morpholino (splice blocker, 8ng) or control morpholino injected embryos at 30hpf. |