- Title
-
The zebrafish dystrophic mutant softy maintains muscle fibre viability despite basement membrane rupture and muscle detachment
- Authors
- Jacoby, A.S., Busch-Nentwich, E., Bryson-Richardson, R.J., Hall, T.E., Berger, J., Berger, S., Sonntag, C., Sachs, C., Geisler, R., Stemple, D.L., and Currie, P.D.
- Source
- Full text @ Development
|
Muscle degeneration in the dystrophic mutants softm272a, cafteg15a and sapta222a. Birefringence images comparing the loss of striated muscle in mutant embryos at 72 hpf, viewed through a polarising filter as dark patches on the trunk. The distribution and severity of muscle lesions in sof are broadly similar to caf and sap, while wild-type embryos (WT) display no loss of birefringency. PHENOTYPE:
|
|
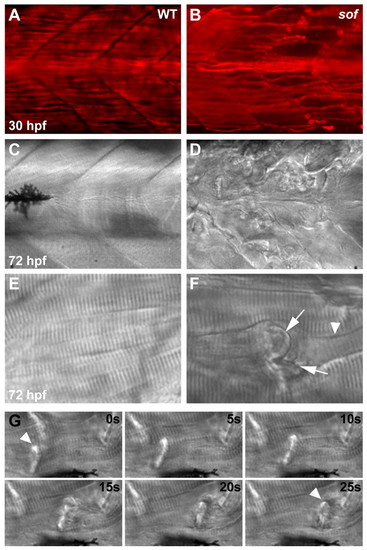
Muscle degeneration in sof mutants results from detachment of myofibres from the vertical myoseptum. Lateral views of wild-type muscle (A,C,E) show fibres spanning the somite between adjacent myosepta, whereas in sof mutants (B,D,F), lesions rapidly develop. (A,B) Whole-mount immunohistochemistry using an antibody against slow myosin demonstrates that, although slow fibres develop normally in sof embryos, by 30 hpf fibres have already detached from the myoseptum. (C-F) DIC images of axial muscle. By 72 hpf, the sof embryo appears severely damaged, with numerous detached fibres scarring the myotome. A magnified view of a single detached fibre (F) shows the characteristic invaginated membrane (arrows) and retraction groove (arrowhead). (G) Retraction of a single fibre (arrowhead), from left of frame to right, in a 72 hpf sof embryo. Still images taken from Movie 1 in the supplementary material, with elapsed time indicated in seconds. EXPRESSION / LABELING:
PHENOTYPE:
|
|
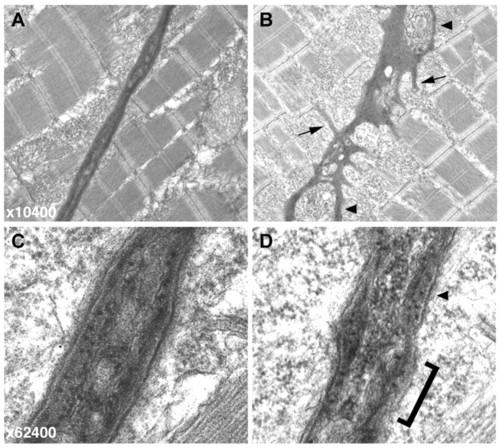
Electron micrographs of the vertical myoseptum reveal a highly irregular MTJ in sof embryos. (A,B) At lower magnification (x10,400), the wild-type myoseptum is compact and linear (A) whereas the sof myoseptum appears severely distorted, even in the absence of fibre detachment (B). Numerous processes extending into the myotome are clearly visible in the mutant (B, arrows), and blisters within the myoseptum are also indicated (arrowheads). (C,D) At higher magnification (x62,400), the smooth, layered structure of the BM at the wild-type MTJ is evident (C). By contrast, the sof BM lacks organisation, with a rough surface (bracketed) and only patches of layering (arrowhead) (D). PHENOTYPE:
|
|
softm272a is an allele of lamb2. (A) softm272a was meiotically mapped to a 2.3 cM region on chromosome 23, delineated by the SSLP markers z13424 and z31657. The non-recombinant marker z31489 was found to reside in an intron of lamb2l. The markers used have been placed on a physical map of chromosome 23, with recombination frequencies shown (number of recombinants/number of meioses). The numbers below the line represent the genetic (top) and physical (bottom) location of markers (Zv7; http://www.ensembl.org). The arrow above the genes indicates transcriptional orientation. (B) The zebrafish lamb2 and lamb2l genes maintain a syntenic relationship with USP19 in human. LAMB2 regions of human chromosome 3 and zebrafish chromosome 23 are displayed, with distances between genes shown (not to scale). Distances were taken from the Ensembl database and Durkin et al. (Durkin et al., 1999). (C) Genomic sequence electropherograms of the mutated region in wild-type, heterozygote and homozygote sof embryos. A single T to C transition was found in exon 3 of lamb2 (asterisk), resulting in the substitution of a proline for a leucine at amino acid 38 of the unprocessed form of Lamb2. The translation is shown below each electropherogram. (D) Domains of Lamb2. The softm272a mutation is in the LN domain, shown in red. Other domains represented are LF domain (blue), EGF-like domain (grey circles) and coiled-coiled domain (grey bar) (Aumailley et al., 2005). (E) Amino acid sequence alignment of the N-terminal region of laminin β2 from zebrafish (Dr), human (Hs), chicken (Gg) and the sole Caenorhabditis elegans β-laminin, LAM-1 (Ce), as well as zebrafish laminin β1, β4 and β2-like. The mutation affects a completely conserved leucine, 21 amino acids from the predicted secreted N-terminal of zebrafish Lamb2. Asterisks denote universally conserved residues. The signal peptide cleavage sites of each sequence were predicted using SignalP3.0 (Bendtsen et al., 2004). (F) Injection of a lamb2 antisense MO precisely phenocopies the loss of birefringency seen in sof homozygous mutants. Top, uninjected wild-type embryo; middle, lamb2 MO3-injected embryo; bottom, sof mutant embryo.
PHENOTYPE:
|
|
Embryonic expression of the lamb2 and lamb2l genes. (A) Lateral view of lamb2 expression in the trunk and tail at 24 hpf. Strong expression is seen in the skeletal muscle. Expression is also seen in the notochord of the tail (arrowhead). (B) Lateral view of lamb2l expression in the trunk and tail at 24 hpf, showing staining in the neural tube (arrowheads) and pronephric duct (arrow). (C,D) Transverse sections of (C) lamb2- and (D) lamb2l-stained embryos, showing distinct expression domains. Neural tube (arrowhead) and pronephric duct (arrow) expression of lamb2l is indicated in D. (E) Dorsal view of head expression of lamb2 at 24 hpf. (F) In a transverse section through the head, at the position indicated by the line in E, lamb2 expression is visible in the cornea, lens and diencephalon. (G) Dorsal view of head expression of lamb2l at 24 hpf. Staining is prominent in the olfactory placode, telencephalon and midbrain. (H) Transverse section of the head of a lamb2l-stained embryo, at the position indicated by the line in G. Expression is seen around the diencephalic ventricle but not in the eye. EXPRESSION / LABELING:
|
|
Analysis of detached fibres indicates that muscle attachment failure occurs external to the sarcolemma. Whole-mount immunohistochemistry of mutant embryos at 72 hpf reveals the presence of dystrophin and β-dystroglycan at the ends of detached fibres. (A-F) Wild-type (A-C) and sof (D-F) embryos stained with anti-dystrophin. (G-L) Wild-type (G-I) and sof (J-L) embryos stained with anti-β-dystroglycan. (A,D,G,J) DIC images, (B,E,H,K) fluorescence images, (C,F,I,L) merged images. Arrows indicate fibre detachments. All panels show lateral views with anterior to the left. dys, anti-dystrophin; bDG, anti-β-dystroglycan. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Two modes of fibre detachment occur in sof muscle. (A-C) In vivo observation of intact fibres in the wild type (A) in comparison with a retracted fibre (B) and an EFT (arrowhead, C) in sof embryos at 120 hpf. Condensed myofibrils can be seen in the retracted fibre (arrowhead, B), whereas fibres attached to the EFT maintain a normal striated appearance. The vertical myoseptum is indicated by arrows in C. (D-K) Antibody co-staining with anti-dystrophin (E,I) and anti-laminin (F,J) reveals the presence of sarcolemma-BM linkages at EFTs in sof embryos (arrow in K), whereas single detached fibres retain sarcolemma only (arrowheads in K). BM detachment can be seen at the junction of the vertical myoseptum and an EFT (inset in K). (L-O) EFTs seen in lamb2 morphants appear identical to those in sof embryos (arrow in O). (P-R) Time points from the monitoring of a single EFT (bracketed) on the Tg(9.7kb smhyc1:gfp)i104 background, with panels labelled according to the stages at which images were captured. The last 16 hours of this period are depicted in Movie 2 in the supplementary material. (S) Anti-laminin staining of the same somite, in red, overlaid onto the GFP fluorescence image, confirming lesion as an EFT. (T-W) Labelling of fibres adjacent to an EFT (arrow in T) with Rhodamine Dextran illustrates viability of detached fibres over 4 days under load-bearing conditions. New fibres occasionally elongate over the EFT (arrow in W), extending to the myoseptum (dashed lines). Panels are labelled according to the stages at which images were captured. (A-C,D,H,L) DIC images, (E,F,I,J,M,N,P-R) fluorescence images, (G,K,O,S,U,W) merge of fluorescence images, (T,V) merge of DIC and fluorescence images. All panels show lateral views with anterior to the left. dys, anti-dystrophin; lam, anti-laminin. |
|
Sarcolemmal rupture correlates with the mode of fibre detachment in sof muscle. (A-C) Evans Blue is excluded from the myotome of wild-type embryos. (D-F) The dye infiltrates a subset of damaged fibres in sof embryos before complete detachment. The fibre depicted still spans the somite, but the contractile cytoskeleton has collapsed in the centre. (G-I) Evans Blue permeates an EFT (arrow in H) but does not enter the fibres attached to it. (J) Electron micrograph of a retracting fibre in a sof embryo at 72 hpf. The vertical myoseptum is to the left of the fibre. (K) A magnified view of the boxed area in J showing trailing sarcolemma at the newly detached tip of the fibre (arrows). (A,D,G) DIC images, (B,E,H) fluorescence images, (C,F,I) merge of DIC and fluorescence images. All panels show lateral views with anterior to the left. PHENOTYPE:
|
|
Expression of wild-type lamb2 in muscle fails to prevent detachment in sof mutant embryos. (A,B) Mosaic expression of the control EosFPtd construct in wild-type (A) and sof (B) embryos. (C,D) Mosaic expression of the rat Lamb2-GFP fusion construct in wild-type (C) and sof (D) embryos. Detaching fluorescent fibres (arrows) can be seen in B and D. All embryos at 72 hpf. |
|
Histology of the sof mutant eye is normal. (A) Lateral views of the head of wild-type sibling (top) and mutant (bottom) embryos at 72 hpf. (B) Transverse section of a wild-type sibling eye. (C) Transverse section of a sof mutant eye. The mutant eye appears normal, with no disruption of basement membranes or lens malformation, taking into account sectioning artefacts. Sections were stained with Hematoxylin and Eosin. |
|
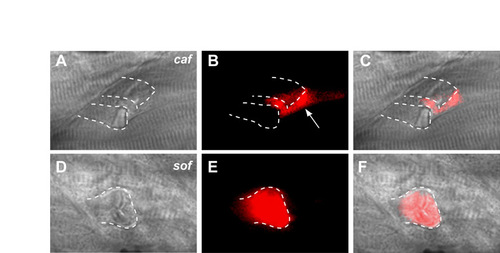
Difference in sarcolemmal pathology between sof and caf. (A-C) A direct comparison between caf and sof mutant embryos confirms that detached fibres in caf remain impermeable to Evans Blue, with the dye leaching into the space created by adjacent retracting fibres (arrow in B). (D-F) By contrast, Evans Blue readily enters a retracted fibre in a sof embryo, with the detached membrane displaying a faceted appearance. (A,D) DIC images; (B,E) fluorescence images; (C,F) merge of DIC and fluorescence images. Dotted lines in all panels demarcate the outline of detached fibres; all panels show lateral views with anterior to the left. |
|
Birefringence of sof;caf double mutants demonstrates that caf is epistatic to sof. Birefringence images comparing the loss of striated muscle in mutant embryos at 120 hpf. The degeneration seen in sof;caf double mutants is not significantly worse than that seen in either single mutant. Wild-type (WT) embryos display no loss of birefringency at 120 hpf. |
|
Analysis of fibre detachment in sof;caf double mutants. (A-H) Antibody co-staining with anti-dystrophin (dys) and anti-laminin (lam) in mutant embryos at 120 hpf. (A,B) Wild-type sibling; (C,D) sof mutant embryo; (E,F) caf mutant embryo; (G,H) sof;caf double mutant embryo. Ectopic fibre termination (EFT)-like structures are seen in sof mutants (arrow in D), whereas detached fibres in caf and sof;caf embryos display only fragmentary basement membrane staining (arrows in F,H). (A,C,E,G) DIC images; (B,D,F,H) merge of fluorescence images. All panels show lateral views with anterior to the left. |

Unillustrated author statements PHENOTYPE:
|