- Title
-
How to make a teleost adenohypophysis: Molecular pathways of pituitary development in zebrafish
- Authors
- Pogoda, H.M., and Hammerschmidt, M.
- Source
- Full text @ Mol. Cell. Endocrinol.
|
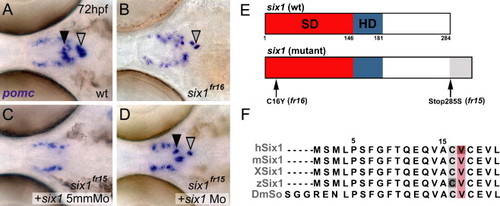
Isolation of two anti- or neomorphic six1 alleles that disrupt pomc expression in the pituitary. (A–D) pomc expression in wild-type (A), six1fr16 mutants (B), six1fr15 mutants injected with a six1 5-mismatch control MO (C), and in a six1fr15 mutant rescued by six1 MO injection (D). The black arrowhead marks pomc expression of the aPD, the grey arrowhead marks pomc expression in the PI. The unaffected bilateral pomc expression domains represent cells of the hypothalamic arcuate nucleus, which in mammals is involved in the regulation of feeding behavior and energy homeostasis. Images show dorsal views on the head region between the eyes, anterior is to the left. Frequencies of obtained phenotypes for the different injection experiments were as follows: fr15: uninjected control = 15 embryos with mutant phenotype/65 evaluated embryos (expected ration 1/4); injection of six1 MO = 3/71; injection of six1 5 mm MO 11/48; fr16: uninjected control = 16/60; plus six1 MO = 4/74; plus six1 5 mm MO 13/56. (E) Schematics of wild-type (top) and mutant (bottom) versions of Six1. Note the C-terminal extension (grey) of the Six1fr15 allele. The six1fr16 mutation converts a cysteine to a tyrosine within the Six domain at amino acid position 16. (F) Sequence alignment of the N-terminal 21 amino acid residues of the human, mouse, frog, zebrafish and Drosophila Six1/So proteins. The highly conserved cysteine residue, which is replaced by a tyrosine in six1fr16, is highlighted in grey. Note the adjacent highlighted valine residue (in salmon), which is affected in an antimorphic allele of human Six1. Abbreviations: SD, six domain (in red); HD, homeodomain (in blue); h, human; m, mouse; X, Xenopus laevis; z, zebrafish; Dm, Drosophila melanogaster. EXPRESSION / LABELING:
PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|
Reprinted from Molecular and Cellular Endocrinology, 312(1-2), Pogoda, H.M., and Hammerschmidt, M., How to make a teleost adenohypophysis: Molecular pathways of pituitary development in zebrafish, 2-13, Copyright (2009) with permission from Elsevier. Full text @ Mol. Cell. Endocrinol.