- Title
-
MicroRNAs are essential for development and function of inner ear hair cells in vertebrates
- Authors
- Friedman, L.M., Dror, A.A., Mor, E., Tenne, T., Toren, G., Satoh, T., Biesemeier, D.J., Shomron, N., Fekete, D.M., Hornstein, E., and Avraham, K.B.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
miRNAs 15a and 18a are expressed in the zebrafish inner ear and miR-15a-1 or miR-18 morphant phenotypes include inner ear defects and reduced HC numbers. (A) ISH shows expression at 48 hpf: miR-183 served as a positive control (Left) for expression in HCs in the saccular macula (SM), utricular macula (UM), and lateral crista (LC). miR-15a was expressed in lateral line neuromasts (NM) and throughout the inner ear, including SCs and canal pillars (CP), and miR-18a expression was strong in the UM and adjacent cells. Dorsal is up, lateral is right. (B–D) Otocysts shown after treatment with MMO-miR-15a-1 or MMO-miR-18a (see Fig. S3). Injection with a standard MO that is not expected to target any known zebrafish RNA serves as a negative control (Left) to emphasize defects in canal pillars, otoliths (oto), and number of HCs in the other morphants. (B) Otocysts at 55 hpf. Anterior is left, dorsal is up. (C) UMs at 55 hpf. Confocal image stacks of HCs viewed en face from the ventral side. HC numbers are listed for each sample. Anterior is left, medial is up. (D) Otocysts at 30 hpf. Confocal image stacks show that morphants have differentiated the earliest HCs, called tether cells (red), at the anterior (arrow) and posterior poles. Primary cilia, labeled with acetylated tubulin, project into the lumen of the otocyst (green dots) but are substantially elongated for tether cells; axons also stain with this antibody. Anterior is left, dorsal is up. [Scale bars: (A), 20 μm; (B), 100 μm; (C), 10 μm; (D), 20 μm.] |
|
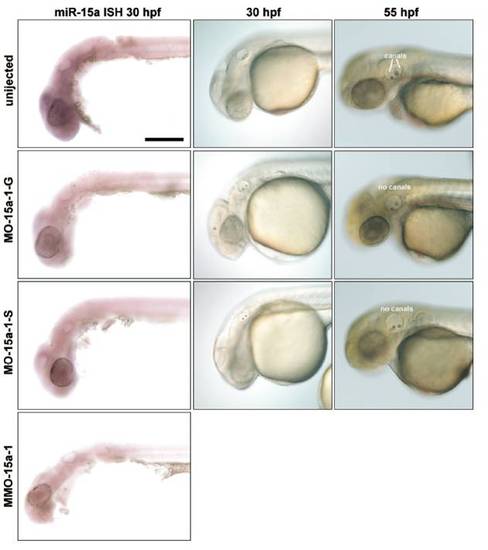
Morpholino knockdown of miR-15a-1 using different MOs. (Left) Thirty-hpf embryos probed with miR-15a locked nucleic acid (LNA) probe to detect mature miRNAs. (Scale bar, 200 μm.) (Middle) Nomarski images of embryos at 30 hpf show presence of 2 otoliths in the inner ear; MMO-15a-1 morphants are indistinguishable from uninjected embryos (data not shown). (Right) Nomarski images of embryos at 55 hpf showing that miR-15a-1 morphants fail to show proper initiation of semicircular canal formation (image of MMO-15a-1 morphant ear at this stage is shown in Fig. 4B and Fig. S6B). Many embryos treated with MO-15a-1-S look normal at this stage; the embryo shown is representative of fewer than 10% that show the canal phenotype. |
|
Morpholino knockdown of miR-18a using different MOs. (Left) Thirty-hpf embryos probed with miR-18a LNA probe to detect mature miRNA. (Scale bar, 200μm.) (Middle) Nomarski images of embryos at 30 hpf show either absent or smaller anterior otoliths in 18a morphants. MO-18a-Sdi morphants are either normal (data not shown) or have smaller anterior otoliths; they typically do not have a specific loss of the anterior otoliths. (Right) Nomarski images of embryos at 55 hpf showing that the phenotype of the anterior otoliths persists, although in most morphants the canals form normally. An exception are the small fraction of MO-miR-18a-Sdi morphants that continue to show reductions in anterior otolith size, which is correlated with a more general loss of canal pillar outgrowth as shown. |
|
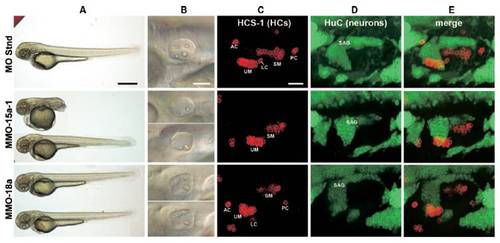
Defects in body and inner ear morphology in zebrafish morphant embryos. MMOs against specific miRNAs were injected to 1-celled embryos and the phenotype 55 h later is presented. A "standard" morpholino (MO Stnd) with a sequence that is not expected to target any known zebrafish RNA served as a negative control for unspecific toxicity. (A) Body phenotypes shown at low magnification.Arange of body types was apparent in miR-15a-1 morphants, including complete tail truncation as the most severe outcome (panel MMO-15a-1). miR-18a morphants had normal bodies (panel MMo-18a), except when MOs were used at very high doses (data not shown). (Scale bar, 500 μm.) (B) Higher power views of the inner ears from the same embryos as those shown on column (A), photographed with Normarski optics. These are the same images shown in Fig. 4. (Scale bar, 100μm.) (C–E) Defects in the sensory organs and ganglia of the inner ears from miR-morphant zebrafish. Confocal image slices were obtained from lateral views of the inner ear and were collapsed as Z-projections to view hair cells (labeled with HCS-1 antibody; column C) and neurons (labeled with HuC antibody; column D) associated with the inner ear. Column (E) presents merged photos. Embryos injected with standard MO showed the normal distribution of 2 maculae, 3 cristae, and a comma-shaped statoacoustic ganglion (SAG). Unlabeled hair cell clusters are cranial neuromasts. 15a-1 morphants had smaller maculae and were missing the 3 cristae. 18a morphants had smaller maculae (notice the utricular macula assumes a curved distribution of hair cells). Abbreviations: AC, anterior crista; LC, lateral crista; PC, posterior crista; SAG, statoacoustic ganglion; SM, saccular macula; UM, utricular macula. (Scale bar, 30 μm.) |