- Title
-
Synaptojanin1 is required for temporal fidelity of synaptic transmission in hair cells
- Authors
- Trapani, J.G., Obholzer, N., Mo, W., Brockerhoff, S.E., and Nicolson, T.
- Source
- Full text @ PLoS Genet.
|
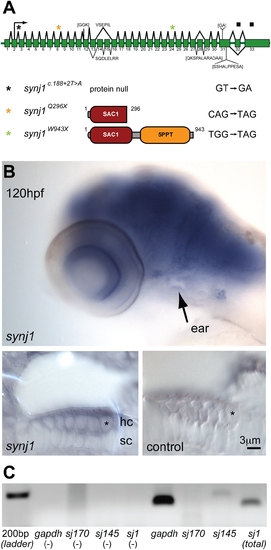
synj1 is mutated in comet mutants. A) Splice diagram of the zebrafish synj1 gene indicating alternate splicing of exons 13, 14 and 15, which can lead to the inclusion or exclusion of other indicated sequences (brackets). The diagram was assembled from collective synj1 sequencing data. Three new mutations of synj1 include deletion of the translation start site (donor splice site inactivation), and nonsense mutations, resulting in truncations at amino acid 296, or amino acid 943. A color-coded asterisk above the splice diagram indicates the position of the respective mutation in the transcript, and the expected protein product is depicted after the allele identifier, followed by the mutated nucleotide sequence. B,C) Expression of synj1 in inner ear and neuromast hair cells. B) synj1 expression pattern in the developing larva as shown by in situ hybridization. At 120 hpf, synj1 is expressed at high levels in the brain, eye and inner ear (arrow; upper panel). Hair-cell specific expression (asterisk) was observed in transverse thick sections of the ear at 100x magnification; both anti-sense synj1 and sense control label are shown (left and right lower panels, respectively). C) RT-PCR for synj1 from isolated neuromast hair-cells amplified the short (synj1-145) isoform. The panel depicts, from left to right, four control reactions (no template) and four hair-cell mRNA template reactions. gapdh mRNA was used as a positive control (band at 151 bp). Specific primer pairs were used to selectively amplify the long and short isoforms sj170 (101 bp expected band size) and sj145 (200 bp), respectively. The sj1 indicates total synj1 mRNA (140 bp). |
|
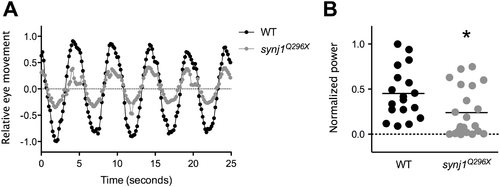
The vestibulo-ocular reflex is reduced in synj1 mutants, indicating a balance defect. Shown in (A) are average relative eye movements of both eyes in wild type (WT; black circles) and synj1Q296X mutant (grey circles) larvae in response to 0.2 Hz stimulation (n>9). B) Plot of power at 0.2 Hz for both eyes for each individual WT (black circles) and mutant (grey circles) larva shown in (A). All values were normalized to the largest WT value. The mean power is represented by the black horizontal lines. PHENOTYPE:
|
|
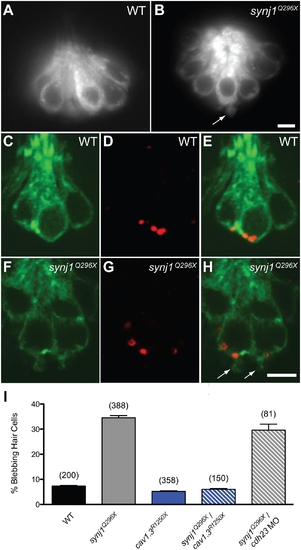
Basal blebbing in synj1Q296X hair cells occurs near synaptic ribbons and is Ca2+-dependent. FM 1–43 labeling of wild-type (A) and synj1Q296X (B) neuromast hair cells. Note the bleb or membrane protrusion at the base of a mutant cell (arrow). Single optical sections of membrane-targeted EGFP (Tg(brn3c:mgfp)) expressed in wild-type (C) and mutant (F) hair cells. D, G) Ribeye-b immunolabel (red staining) of the same neuromasts, indicating ribbons. E, H) Overlays of membrane-targeted EGFP and Ribeye-b label. Arrows in panel H indicate blebs near the ribbon in mutant hair cells. I) Basal blebbing was Ca2+-dependent. Blebbing occurred minimally in neuromast hair-cells of wild-type larvae (black bar). In contrast, blebbing was significantly increased in synj1Q296X mutant hair cells (grey bar). Blebbing was minimal in cav1.3R1250X mutant hair cells (blue bar), as well as cav1.3R1250X/synj1Q296X double mutant hair cells (blue stripe bar). Blebbing still occurred in synj1Q296X mutants that were transduction-deficient by morpholino-mediated cadherin23 knockdown (grey stripe bar). Numbers in parentheses above bars indicate number of hair cells examined. Scale bars, 3 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
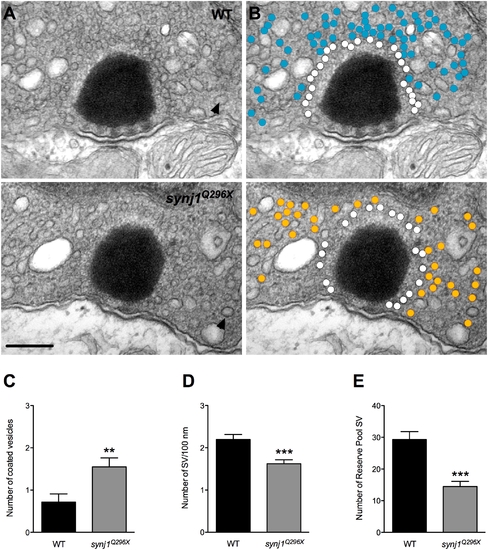
Impaired vesicle recycling at ribbon synapses in mutant synj1 hair cells. A) TEM micrographs of neuromast ribbon synapses (upper panel: wild-type ribbon; lower panel: synj1Q296X mutant ribbon). B) Same micrographs as in (A) with white circles denoting tethered vesicles. Blue and orange circles highlight the reserve vesicles in the wild type and mutant synapses, respectively. Note the reduction of reserve pool vesicles in the mutant synapse. Arrow heads indicate examples of large coated vesicles. Scale bar, 200 nm. Number of large coated vesicles (C), tethered vesicles per 100 nm of ribbon perimeter (D), and reserve pool vesicles (E) within 450 nm of ribbon centers in neuromast hair cells (black bars: wild type; grey bars: synj1Q296X). PHENOTYPE:
|
|
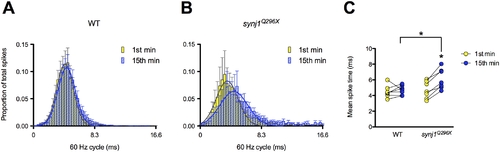
The mean timing of spikes in response to 60 Hz stimulation is delayed in synj1Q296X mutants. A, B) Histograms of 60 seconds of activity for (A) wild-type and (B) synj1Q296X afferent neurons in the first minute (yellow bars) and fifteenth minute (blue bars) of 60 Hz stimulation. The x-axis represents the length of one 60 Hz cycle in milliseconds. Curves were fit to Gaussian distributions (yellow and blue solid lines). C) Mean spike times of activity during 60 seconds of stimulation for individual wild-type and synj1Q296X mutants represented in (A). Lines connect the 1st (yellow circles) and 15th (blue circles) minute of stimulation for each larval recording. Following 15 minutes of sustained 60 Hz stimulation there was a significant delay in spike time compared to the first minute of stimulation in synj1Q296X as well as compared to wild-type after 15 minutes of stimulation. PHENOTYPE:
|
|
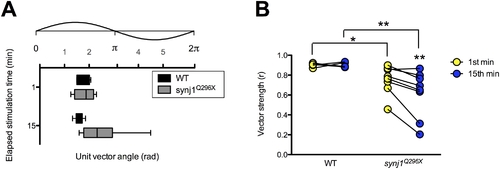
The fidelity of phase locking is decreased in synj1Q296X larvae. A) Box plot representing phase angles from the unit vectors described by all spikes in the 1st and 15th minute of 60 Hz stimulation from wild-type (black bars) and synj1Q296X (grey bars) larvae. Above the box plots is the corresponding 60 Hz stimulus cycle with phase scale in radians. B) Individual wild-type and synj1Q296X mutant vector strength (r) values for the 60 seconds of activity described in (A). Lines connect the 1st (yellow circles) and 15th (blue circles) minute of stimulation for each larval recording. Mutant vector strength was not only reduced at both time points compared to wild-type, but also from the 1st to the 15th minute of stimulation. PHENOTYPE:
|
|
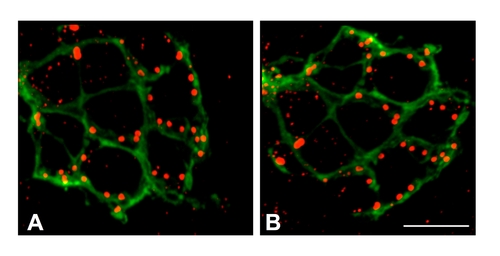
Synaptic ribbons in synj1Q296X hair cells are localized to basolateral regions adjacent to lateral line nerve fibers. Ribbons in wild-type (A) and synj1Q296X (B) neuromasts were labeled with anti-Ribeye b antibody (red staining). Nerve fibers were visualized using GFP fluorescence stably expressed by a neurod promoter (green staining). Shown are maximum projections of confocal sections from a top down view. Scale bar, 3 μm. |
|
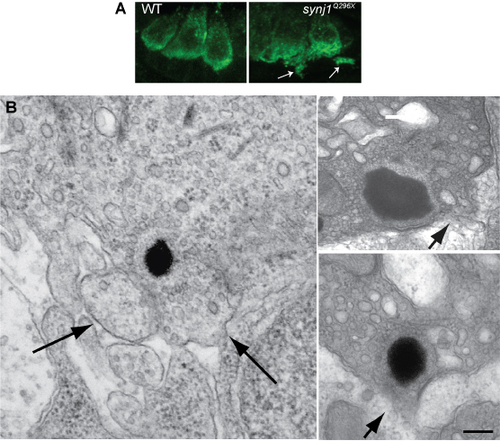
Examples of loss of integrity of the plasma membrane near mutant synj1Q296X ribbons. (A) Confocal optical sections of inner ear hair cells labeled with anti-Vglut3 antibody in wild type and synj1Q296X larvae. (B) Left panel, blebbing seen in an synj1Q296X inner ear hair cell at the TEM level. Right panels, two ribbons in synj1Q296X neuromast hair cells. Arrows indicate membrane protrusions or areas that appear to the disintegrating. Scale bar, 125 nm in left panel; 100 nm in top right panel; 200 nm in lower right panel. |