- Title
-
A functional interaction between Irx and Meis patterns the anterior hindbrain and activates krox20 expression in rhombomere 3
- Authors
- Stedman, A., Lecaudey, V., Havis, E., Anselme, I., Wassef, M., Gilardi-Hebenstreit, P., and Schneider-Maunoury, S.
- Source
- Full text @ Dev. Biol.
|
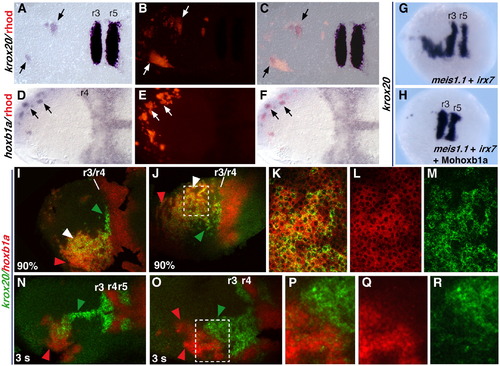
irx1b and irx7 are required for the expression of krox20 in r3. Embryos uninjected (ni; A, E, I, K, M) or injected with the morpholino against irx7 (Mo7; B,F) or against irx1b (Mo1b; C, G), coinjected with both morpholinos (Mo1bMo7) (D, H, J, L, N) or injected with Mo1b, Mosp7 and irx7 and gfp RNAs (O, P), were stained by ISH with probes indicated on the left and colour-coded. Panels A–D are lateral views of whole embryos, and panels E–P are dorsal views of flat-mounted embryos. Panel P is a high magnification of part of the hindbrain of a partially rescued morphant, which shows that krox20 expression in r3 is restored specifically in gfp and irx7 expressing cells (arrows). Rostral is toward the left. The embryonic stage is indicated at the bottom-right of each picture. Arrowheads in panels J, L, N indicate the absence of krox20 expression at the position of r3 in double Mo1bMo7 morphants. EXPRESSION / LABELING:
PHENOTYPE:
|
|
irx1b and irx7 are required to pattern the anterior hindbrain. (A–R) Embryos uninjected (A,C,E,G,I,K,M,O,Q) or coinjected with Mo1b and Mo7 (B, D, F, H, J, L, N, P, R) were stained by ISH with probes indicated on the left and colour-coded. Dotted lines in panels A and B indicate the AP length of the r1–r4 region. Arrowheads in panels B, F, H point to reduced expression domains of hoxb1a, cyp26c1 and cyp26b1, respectively. (S, T) Isl1-GFP transgenic embryos uninjected (S) or coinjected with Mo1b and Mo7 (T). The motoneurons of cranial nerves V (trigeminal, born in r2 and r3) and VII (facial, born in r4 and migrating into r6–r7) and the rhombomeres (r) are indicated. The dotted ovals show the position of the otic vesicle. All pictures are dorsal views of the hindbrain region of flat-mounted embryos, with rostral toward the left. The embryonic stage is indicated at the bottom-right of each picture. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Coinjection of irx7 and meis1.1 RNAs leads to ectopic expression of krox20 in the anterior neural plate. Embryos uninjected (A) or injected with RNAs for irx7 (B), meis1.1 (C),meis1.1 and irx7 (D, E, H, I), meis2.1 (F), meis2.1 and irx7 (G) were stained by ISH with probes indicated on the left and colour-coded. Panels A–H are wild type embryos, and I is a vhnf1 homozygous embryo. All pictures show dorsal views of flat-mounted (A–E, H, I) or whole (F, G) embryos, with anterior toward the left. All embryos are at the 5 s stage. |
|
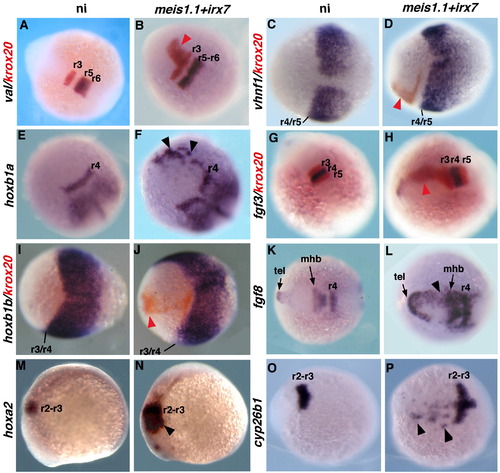
Coinjection of irx7 and meis1.1 RNAs leads to ectopic expression of anterior, but not of posterior, hindbrain markers. Embryos uninjected (A, C, E, G, I, K, M, O) or coinjected with meis1.1 and irx7 RNAs (B, D, F, H, J, L, N, P) were processed for ISH with the probes indicated on the left and colour-coded. A–L, O, P show dorsal views of whole embryos with rostral toward the left (C, D, H, I–L, O, P) or toward the top left (A, B, E, F, G). M, N are lateral views of whole embryos with rostral toward the left. Stages are 5 s for A, B, E, F, G, H, K, L, O, P; 15 s for M,N and 100% epiboly for C, D, I, J. Black and red arrowheads point to ectopic gene expression domains (colour-coded). tel: telencephalon. |
|
Mechanisms of krox20 activation downstream of irx7 and meis1.1. (A–F) Host embryos processed for rhodamine (B, E) and krox20 (A) or hoxb1a (D) staining, after transplantation of cells form donor embryos injected with rhodamindextran, irx7 and meis1.1. Panels C and F are merged images of panels A and B, or panels C and D, respectively. (G, H) Embryos injected with irx7 and meis1.1 RNAs and with (H) or without (G) a morpholino for hoxb1a (Mohoxb1a). (I–R) Embryos coinjected with irx7 and meis1.1 RNAs and processed for fluorescent ISH. K–M and P–R are higher magnifications of the region squared in J and O, respectively, showing krox20 staining (green, M,R), hoxb1a staining (red, L, Q) or both (merge, K, P). All pictures are dorsal views, with anterior to the left, of flat-mounted (A–F and I–R) or whole (G, H) embryos. Probes are indicated on the left and colour-coded. All embryos are at the 5 s stage, except when otherwise stated at the bottom left of the picture. In panels I–R, green, red and white arrowheads point to cells showing ectopic expression of krox20 only, hoxb1a only or both, respectively. |
|
Irx7 activates and binds in vivo the krox20 cis-regulatory element C. (A–D) Embryos injected with eltC:gfp DNA alone (A, C) or with eltC:gfp DNA and irx7 + meis1.1 RNAs (B, D) and analysed for GFP activity (A, B) or by double krox20/gfp ISH (C, D) at 24 hpf. (E–H) Tg(eltC:gfp) embryos uninjected (E, F), injected with irx7 + meis1.1 RNAs (G) or injected with Mo1b and Mo7 (H) and analysed for GFP activity at 24 hpf (E) or by double krox20/gfp ISH at 3 s (F–H). In embryos shown in panels F–H, gfp (purple) and krox20 (red) expression domains perfectly overlap, resulting in a brown staining. Dotted ovals in panels A, B and E indicate the position of the otic vesicle. Arrows in panels D and G indicate regions of ectopic coexpression of gfp and krox20. (I, J) PCR amplification of DNA fragments from the krox20 locus: element C (- 41 kbp from the krox20 transcription start site), a 422 bp genomic fragment (- 22,4 kbp), and the core promoter (- 114 bp) on DNA obtained by ChIP with an Irx7 antibody on uninjected embryos (I) or with a anti-Myc antibody on meis1.1myc RNA injected embryos (J). Ab: antibody; preI: preimmune serum. Input: DNA before immunoprecipitation. The embryonic stages are indicated at the bottom right in panels A–H and on the left in panels I, J. EXPRESSION / LABELING:
|
|
Anti-Caspase 3 staining of apoptotic cells in Mo1bMo7 morphants. Control embryos (A) and Mo1bMo7Mop53 morphants (B–D) were stained with the otx2 and vhnf1 probes (purple staining) and with the anti-Caspase 3 antibody (brown staining). The black lines indicate the length of the anterior hindbrain, the region lying between the mhb and r5. Black arrows in A and B point to some of the Caspase 3 positive-cells. In the same experiment, some embryos were let to develop until the 5 s stage and were checked for krox20 expression in r3 as a control of the knock-down efficiency. In all of the embryos analysed (n = 10), krox20 expression in r3 was lost. |
|
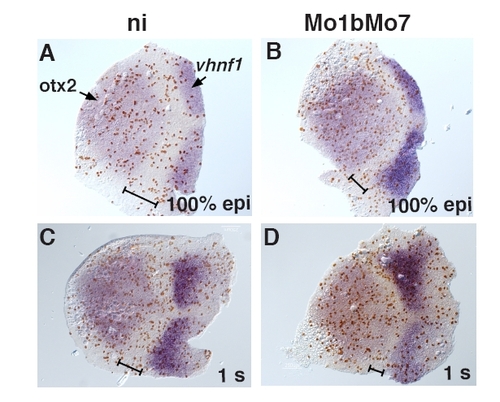
Cell proliferation in Mo1bMo7 morphants. Embryos uninjected (ni, A, C) or injected with Mo1b and Mo7 (B, D) were fixed at 100% epiboly (A, B) or 1 s (C, D) and subjected to ISH with probes for otx2 and vhnf1 and IHC for the mitosis marker phosphorylated-Histone H3 (H3P). The number of mitotic cells per surface unit was counted in the anterior hindbrain, defined as the territory located between the otx2 and vhnf1 expression domains (black line). Anterior is toward the left. |
|
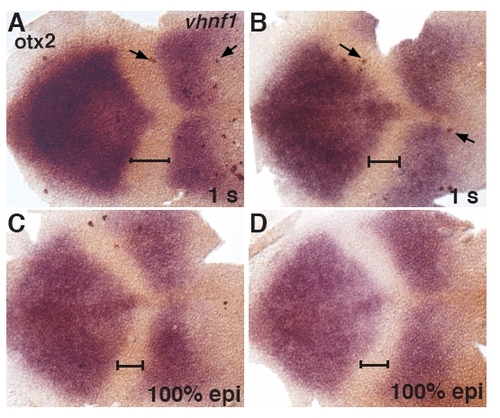
Expression domains of irx7 and meis1.1 overlap in the r3–r4 region and in the midbrain. Zebrafish embryos at the tailbud (A) and 1 s (B) stages were stained by ISH with probes indicated on the left and colour-coded. Dorsal view of a flat-mounted embryos, with rostral toward the left. |
|
Irx7 and Meis1.1 do not interact in vitro. A) Detection of Irx7 in zebrafish embryos by the Irx7 antibody. Nuclear protein extracts from 100% epiboly zebrafish embryos were submitted to Western-blot performed with the Irx7 antibody, or with the preimmune serum. In uninjected embryos (ni), Irx7 is detected by the antibody with an apparent molecular weight of 43 kDa, but not by the preimmune serum. The specificity of the antibody is confirmed by the absence of a band in Western blots performed on Mo7 injected embryos. B) Coimmunoprecipitations of a HA-tagged Irx7 protein and a Myc-tagged Meis1.1 protein, produced in transfected COS7 cells. Irx7HA and Meis1.1Myc were mixed and immunoprecipitated with antibodies specific for either Irx7 or Myc. The precipitation products were assayed by Western-blotting for Irx7 (upper panel) and Myc (lower panel). Irx7 and Meis1.1 do not coimmunoprecipitate. C) Anti-Myc coimmunoprecipitation of in vitro translated proteins, using the reticulocyte lysate system. Irx7 was translated together with Pbx4Myc (lanes 1 and 2), Meis1.1Myc and Pbx4 (lanes 3 and 4), or Meis1.1Myc and LacZ as a control (lanes 5 and 6) in the presence of 35Smethionine, and analysed by autoradiography either directly or following an anti-myc co-immunoprecipitation. Pbx4 protein coimmunoprecipitated with Meis1.1Myc (black rectangle) whereas Irx7 was not coimmunoprecipitated. Note that the Irx7 protein produced by in vitro translation is present as two bands. Ab: antibody; preI: preimmune serum. EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 327(2), Stedman, A., Lecaudey, V., Havis, E., Anselme, I., Wassef, M., Gilardi-Hebenstreit, P., and Schneider-Maunoury, S., A functional interaction between Irx and Meis patterns the anterior hindbrain and activates krox20 expression in rhombomere 3, 566-577, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.