- Title
-
Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants
- Authors
- Sukumaran, S., and Perkins, B.D.
- Source
- Full text @ Vision Res.
|
Transmission electron micrographs of transverse sections along the dorsal–ventral axis of wild type, ift57, ift88, and ift172 mutant zebrafish at 60 hpf. (A) In wild type retinas, few outer segments were observed at low magnification. The interphotoreceptor space was seen between the inner segments and the pigment epithelium (short arrows). Photoreceptor nuclei (n) were easily identified. (B and C) At higher magnification, short outer segments with loosely organized disk membranes were seen. Connecting cilia and primitive cilia (arrows) projected from the inner segment above the mitochondria (m) and nuclei (n). (D) ift57 mutants appeared similar to wild type retinas at low magnification. (E and F) High magnification of ift57 mutants revealed outer segments with stacked disk membranes and connecting cilia (arrow) that were located distal to the mitochondria (m) and nuclei (n). (G) An electron micrograph of an ift88 mutant looked similar to wild type and the interphotoreceptor space remained present (short arrows). (H and I) Outer segments were never observed in ift88 mutants and disorganized membrane structures (arrowheads) were seen at the apical surface of the inner segment, above the mitochondria and nuclei. (J) A low magnification image of ift172 mutants showed few differences from wild type. (K and L) High magnification electron micrographs revealed disorganized membrane structures (arrowheads) located deep within the inner segment below the mitochondria (m), as well as at the apical surface. Scale bars = 4 μm (A, D, G, and J), and 400 nm (B, C, E, F, H, I, K, and L). |
|
Transmission electron micrographs of transverse sections along the dorsal–ventral axis of wild type, ift57, ift88 and ift172 mutant zebrafish at 72 hpf. (A) Electron micrograph of a wild type embryo showed photoreceptor outer segments (arrows) that were easily observed at low magnification. (B and C) Higher magnification showed that outer segment disk membranes are well organized and the connecting cilium (arrows) can be seen. These cilia were approximately 300 nm in width and 500 nm in length. Mitochondria (m) were observed in the apical inner segment. (D) In ift57 mutants, outer segments were observed at low magnification. (E and F) At higher magnification, the disk membranes were well ordered but the outer segments appear somewhat disheveled compared to wild type. Arrows denote connecting cilia. (G) In ift88 mutants, no outer segments were seen and disorganized membrane structures (arrowhead) were seen near the nuclei (n). (H and I) Higher magnification images revealed the interphotoreceptor space (ips) between the RPE and the mitochondria (m), as well as membrane structure (arrowheads) located basal to the mitochondria. (J) Electron micrograph of an ift172 mutant also showed no outer segments. (K and L) Disorganized membrane structures (arrowhead) running parallel to the plasma membrane were seen basal to the mitochondria (m). A centriole (asterisk) that may be part of a mispositioned basal body was also seen several hundred nanometers away from the apical surface and below a mitochondria (m). Scale bar = 4 μm (A, D, G, and J), and 400 nm (B, C, E, F, H, I, K, and L). PHENOTYPE:
|
|
Transmission electron micrographs of transverse sections along the dorsal–ventral axis of wild type, ift57, ift88, and ift172 mutant zebrafish at 96 hpf. (A–D) Low magnification images revealed longer outer segments (os) in wild type embryos. Photoreceptor outer segments were present in ift57 mutants but were significantly shorter than wild type. Mitochondria (m) and nuclei (n) appear to be normal in the mutants. No outer segments were present in ift88 and ift172 mutants. Nuclei of ift88 and ift172 mutants were less elongated than wild type and pyknotic nuclei (asterisk, C) were occasionally observed. (E–H) At higher magnification, the disk membranes of wild type and ift57 mutant outer segments were highly organized. Arrow indicates connecting cilium. Numerous mitochondria (m) were seen in the apical inner segments of wild type and mutant photoreceptors. Scale bars = 1 μm (A–D), and 500 μm (E–H). PHENOTYPE:
|
|
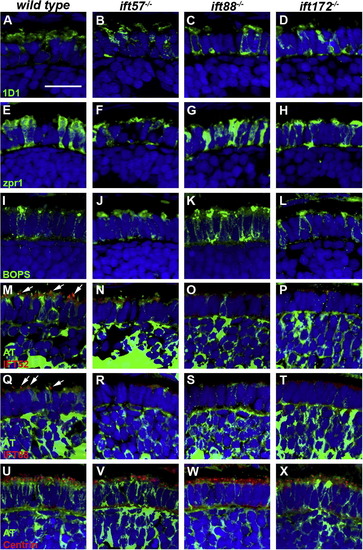
Immunohistochemical analysis of wild type, ift57, ift88, and ift172 mutant zebrafish at 60 hpf. (A–D) 1D1 (green), a marker for rhodopsin, localized to the outer segment region in wild type and ift57 mutants. Strong mislocalization to the inner segment was also observed in ift57 mutants, as well as ift88, and ift172 mutants. (E–H) Zpr1 (green), a label for red/green double cones gave an elongated, columnar morphology in wild type animals. Cone morphology was disheveled in the three mutants. (I–J) Blue opsin (BOPS) was seen in small foci of wild type photoreceptors. Similar staining was seen in ift57, ift88, and ift172 mutants. (M–P) IFT52 (red) co-localized with acetylated tubulin (green) in the connecting cilia of wild type animals (arrows). No such staining was observed in ift57, ift88, and ift172 mutants. (Q–T) IFT88 (red) also showed apical localization in wild type animals (arrows). The staining partially overlapped with acetylated tubulin (green). No such staining was observed in IFT mutants. (U–X) The basal body marker centrin (red) localized to the apical surface of the inner segment. Acetylated tubulin (green) denotes microtubules. Centrin was seen in wild type and all IFT mutants (arrows). In all images, the tissues were counterstained with DAPI (blue). Scale bar = 20 μm. |
|
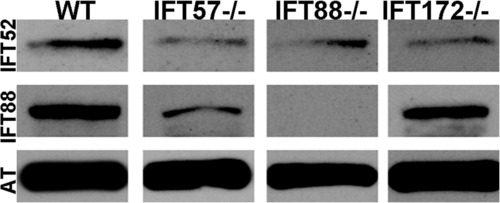
Western blot analysis of whole wild type and IFT mutant embryos at 60 hpf. Immunoreactivity against IFT52 protein (top row) was detected in wild type, ift57, ift88, and ift172 mutants. Immunoreactivity against IFT88 protein (middle row) was seen only in wild type, ift57 and ift172 mutants, thus showing that the ift88 mutant is null. Acetylated tubulin (AT) served as a control (bottom row). EXPRESSION / LABELING:
|
|
Immunohistochemical analysis of wild type, ift57, ift88 and ift172 mutant zebrafish at 72 hpf. (A–D) 1D1 (green), a marker for rhodopsin, localized to the outer segment region in wild type and ift57 mutants. Strong mislocalization to the inner segment was observed in ift57 mutants, as well as ift88, and ift172 mutants. (E–H) Zpr1 (green), a label for red/green double cones gave an elongated, columnar morphology in wild type animals. The columnar morphology was disrupted in the three mutants. (I–J) Blue opsin (BOPS) was localized to putative outer segments of wild type photoreceptors, although some mislocalization was seen. Staining was observed in small the apical region of IFT mutant photoreceptors but strong mislocalization was seen in the ift88 and ift172 mutants. (M–P) IFT52 (red) co-localized with acetylated tubulin (green) in the connecting cilia of wild type (arrows). No such staining was observed IFT mutants. (Q–T) IFT88 (red) also showed strong apical localization in wild type embryos (arrows). The staining partially overlapped with acetylated tubulin (green). No staining was seen in the IFT mutants. (U–X) The basal body marker centrin (red) localized to the apical surface of the inner segment. Acetylated tubulin (green) denotes microtubules. In all images, the tissues were counterstained with DAPI (blue). Scale bar = 20 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
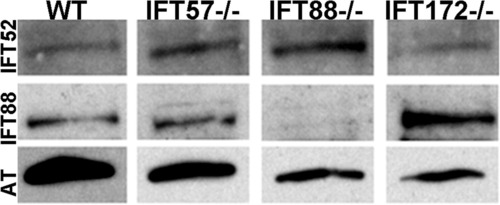
Western blot analysis of whole wild type and IFT mutant embryos at 72 hpf. Immunoreactivity against IFT52 (top row) were detected in wild type and mutants at 72 hpf, confirming the expression of these subunits. IFT88 immunoreactivity (middle row) was not seen in the ift88 mutants but continued to be expressed in other mutants as well as wild type. Acetylated tubulin (AT) served as a control (bottom row). EXPRESSION / LABELING:
|
|
Immunohistochemical analysis of wild type, ift57, ift88 and ift172 mutant zebrafish at 96 hpf. (A–D) 1D1 (green), a marker for rhodopsin, localized to the outer segment region in wild type and ift57 mutants. Strong mislocalization to the inner segment was also observed in ift57, ift88, and ift172 mutants. (E–H) Zpr1 (green), a label for red/green double cones gave an elongated, columnar morphology in wild type animals. Cone morphology was disheveled in the three mutants. (I–J) Blue opsin (BOPS) was localized to the outer segments of wild type photoreceptors. Staining was observed in short outer segments of ift57 photoreceptors but strong mislocalization was seen in the ift88 and ift172 mutants. (M–P) IFT52 (red) co-localized with acetylated tubulin (green) in the connecting cilia of wild type and ift57 mutants (arrows). No such staining was observed in ift88 and ift172 mutants. (Q–T) IFT88 (red) also showed strong apical localization in wild type and ift57 mutants. The staining overlapped with acetylated tubulin (green), in wild type and ift57 animals (arrows). No staining was observed in ift88 and ift172 mutants. (U–X) Centrin (red) localized to the apical surface of the inner segment. Acetylated tubulin (green) denotes microtubules. Centrin was seen in wild type and all IFT mutants. The number of centrin-positive foci was reduced in ift88 and ift172 mutants. Centrin was occasionally observed to be mislocalized in ift172 mutants (Fig. 9X; white arrowheads). In all images, the tissues were counterstained with DAPI (blue). Scale bar = 20 μm. |
Reprinted from Vision Research, 49(4), Sukumaran, S., and Perkins, B.D., Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants, 479-489, Copyright (2009) with permission from Elsevier. Full text @ Vision Res.