- Title
-
The cell adhesion molecule Tag1, transmembrane protein Stbm/Vangl2, and Lamininalpha1 exhibit genetic interactions during migration of facial branchiomotor neurons in zebrafish
- Authors
- Sittaramane, V., Sawant, A., Wolman, M.A., Maves, L., Halloran, M.C., and Chandrasekhar, A.
- Source
- Full text @ Dev. Biol.
|
Expression patterns of tag1, stbm, and lama1 in the hindbrain during FBMN migration. All panels show dorsal views of the hindbrain with anterior to the left. Asterisks mark the otic vesicle in each panel. (A, C) tag1 is expressed in an increasing number of migrating FBMNs (arrowheads) between 19 hpf (A) and 24 hpf (C). Strongest expression is initially found in cells located in r5/r6 (A), and later in cells spanning r5 and r6 (C). (B) In a 24 hpf Tg(isl1:gfp) embryo, FBMNs (arrowheads) are found throughout the migratory pathway from r4 to r7, with their axons (arrow) exiting the hindbrain in r4. (D) At 24 hpf, Tag1 immunostaining labels only cell bodies of FBMNs located in r6 and r7 (arrowhead), and their axons (arrow). (E) stbm is ubiquitously expressed in the neural tube, and in surrounding non-neural tissues. (F) A 60 μm stack shows broad Laminin1 immunostaining in the basal lamina in the ventral neural tube and adjacent non-neural tissues. tg, trigeminal ganglion; ag, acoustic ganglion; pg, posterior lateral line ganglion. Scale bar in A (75 μm for A, C, E); in B, D, F (75 μm). EXPRESSION / LABELING:
|
|
FBMN migration is affected in tag1 morphants. Top panels show dorsal views of the hindbrain with anterior to the left. FBMN cell bodies and axons were visualized in Tg(isl1:gfp) embryos using anti-GFP antibody. (A) In a 36 hpf control (uninjected) embryo, FBMNs (arrowheads) migrate normally into r6 and r7. (B) An embryo injected with a suboptimal dose (6 ng) of tag1 MO exhibits an intermediate phenotype, with many FBMNs (arrowheads) remaining in r4 and others migrating into r6 and r7. (C) In an embryo injected with an optimum dose (12 ng), most FBMNs (arrowhead) fail to migrate tangentially out of r4, but many appear to be displaced into r5. (D) Quantification of the tag1 MO dose–response effect. The green, yellow, and red phenotypic classes correspond to the FBMN migration patterns depicted in panels A–C, respectively. Data from 2–3 experiments; number in parenthesis denotes number of embryos. Scale bar in A (75 μm for A–C). |
|
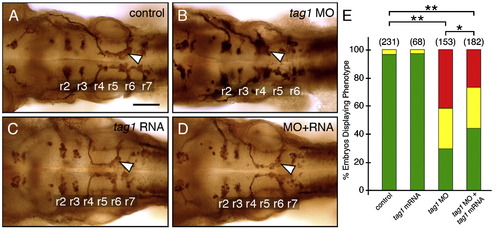
Rescue of the tag1 morphant phenotype. Panels A–D show dorsal views of the hindbrain with anterior to the left. FBMN cell bodies and axons were visualized in Tg(isl1:gfp) embryos using anti-GFP antibody. Arrowheads indicate FBMNs. (A) FBMNs migrate normally in an uninjected control embryo. (B) FBMNs mostly fail to migrate in a tag1 morphant embryo. (C) FBMNs migrate normally in a tag1 mRNA injected embryo. (D) FBMN migration is mostly rescued in an embryo co-injected with tag1 MO and tag1 mRNA. (E) Quantification of the rescue data. The distribution of different phenotypes among the various treatments was analyzed by Pearson's Chi-Square statistics. The differences in the phenotypic distributions between the indicated pairs of samples were highly significant (*, P < 0.01; **, P < 0.001). Data from 4 experiments. Number in parenthesis denotes number of embryos. Scale bar in A (75 μm for A–D). |
|
Neuronal defects in tag1 morphants. Panels A, B, E–J show dorsal views of the head with anterior to the left (anterior at top in G, H). Panels C, D, K and L show lateral views of the head with anterior to the left. tag1 morphants (D, F, and H) were co-injected with p53 MO to minimize non-specific apoptosis (Robu et al., 2007). WT embryos (controls) either uninjected or injected with p53 MO alone exhibited identical phenotypes in the tissues examined. (A, B) Migration of FBMNs (arrowheads) into r6 and r7 (A) is mostly eliminated in a tag1 MO-injected embryo (B). Zn5-labeled dorsal commissural neurons (red) at rhombomere boundaries develop in similar numbers in control and morphant embryos. (C, D) While acridine orange (AO) labeling reveals very few dying cells in a control embryo (C), there are many more dying cells (arrowhead) in a tag1 morphant (D). Asterisk marks otic vesicle. (E) In a control embryo, FBMNs migrate in close proximity to the medial longitudinal fascicle (MLF, arrowhead). Arrow indicates the dorsal longitudinal fascicle (DLF) containing the central (afferent) axons of the trigeminal sensory neurons. (F) In a tag1 morphant, the MLF (arrowhead) and DLF (arrow) appear brighter, but develop normally. (G, H) The trigeminal sensory ganglion (arrowhead) in a control embryo (G) is compact (inset shows lateral view), with prominent peripheral axons (arrowhead). In contrast, the trigeminal ganglion in a tag1 morphant (H) is composed of loosely organized cells (arrowheads, and inset showing lateral view), with peripheral axons (arrow) extending broadly over the head. Asterisks indicate the nucleus of the MLF, with brightly-labeled projections in the morphant, compared to the control embryo. (I, J) Expression of krox20 in r3 and r5 is similar between control and tag1 morphant embryos. (K, L) Ventrocaudal migration of tyrosine hydroxylase (TH)-positive locus coeruleus neurons (arrowhead) into r1 is similar between control and tag1 morphant embryos. Scale bar in D (150 μm for C, D); in E (75 μm for A, B, E–H); in J (75 μm for I–L). EXPRESSION / LABELING:
|
|
Genetic interactions between tag1 and stbm. All panels show dorsal views of the hindbrain with anterior to the left. Tg(isl1:gfp) embryos were fixed at 48 hpf, and processed for immunohistochemistry with zn5 antibody (red) to label dorsal commissural neurons and axons at rhombomere boundaries, and anti-GFP antibody (green) to label FBMNs (arrowheads). (A) FBMNs migrate normally in a control embryo. (B, C) Partial loss of FBMN migration in embryos injected with suboptimal dose of tag1 MO (B) or stbm MO (C). (D) Complete loss of FBMN migration in an embryo injected with suboptimal doses of tag1 and stbm MOs. (E) Partial loss of FBMN migration in a trilobitetc240a (tri) heterozygous (stbm +/-) embryo. (F) Complete loss of FBMN migration in a trilobite heterozygote injected with suboptimal dose of tag1 MO. Scale bar in F (75 μm for A–F). |
|
Genetic interactions between stbm and lama1. All panels show dorsal views of the hindbrain with anterior to the left. Tg(isl1:gfp) embryos were fixed at 48 hpf, and processed for immunohistochemistry with zn5 antibody (red) to label dorsal commissural neurons and axons at rhombomere boundaries (A–F), and anti-GFP antibody (green) to label FBMNs (A–H; arrowheads). (A) FBMNs migrate normally in a control embryo. (B, C) Partial loss of FBMN migration in embryos injected with suboptimal dose of lama1 MO (B) or stbm MO (C). (D) Complete loss of FBMN migration in an embryo injected with suboptimal doses of lama1 and stbm MOs. (E) Partial loss of FBMN migration in a trilobitetc240a (tri) heterozygous (stbm +/-) embryo. (F) Complete loss of FBMN migration in a trilobite heterozygote injected with suboptimal dose of lama1 MO. (G) Greatly reduced FBMN migration in a bashfulb765 homozygous (lama1 -/-) embryo. (H) Complete loss of FBMN migration in a trilobite; bashful double heterozygote. Scale bar in H (75 μm for A–H). |
|
Genetic interactions between tag1 and lama1. All panels show dorsal views of the hindbrain with anterior to the left. Tg(isl1:gfp) embryos (A–D) were fixed at 48 hpf, and processed for immunohistochemistry with zn5 antibody (red) to label dorsal commissural neurons and axons at rhombomere boundaries, and anti-GFP antibody (green) to label FBMNs (arrowheads). Non-transgenic embryos (E, F) were fixed at 30 hpf, and processed for tag1 in situ hybridization to label FBMNs (arrowheads). (A) FBMNs migrate normally in a control embryo. (B, C) Partial loss of FBMN migration in embryos injected with suboptimal dose of lama1 MO (B) or tag1 MO (C). (D) Complete loss of FBMN migration in an embryo injected with suboptimal doses of tag1 and lama1 MOs. (E) Greatly reduced FBMN migration in a bashfuluw1 homozygous (lama1 -/-) embryo. (H) Severe, nearly complete loss of FBMN migration in a putative bashful heterozygote injected with suboptimal dose of tag1 MO. Scale bar in D (75 μm for A–D); in F (75 μm for E, F). |
Reprinted from Developmental Biology, 325(2), Sittaramane, V., Sawant, A., Wolman, M.A., Maves, L., Halloran, M.C., and Chandrasekhar, A., The cell adhesion molecule Tag1, transmembrane protein Stbm/Vangl2, and Lamininalpha1 exhibit genetic interactions during migration of facial branchiomotor neurons in zebrafish, 363-373, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.