- Title
-
Mutation of cGMP phosphodiesterase 6alpha'-subunit gene causes progressive degeneration of cone photoreceptors in zebrafish
- Authors
- Nishiwaki, Y., Komori, A., Sagara, H., Suzuki, E., Manabe, T., Hosoya, T., Nojima, Y., Wada, H., Tanaka, H., Okamoto, H., and Masai, I.
- Source
- Full text @ Mech. Dev.
|
Zebrafish OKR-defective mutants. (A, B) Dorsal (A) and lateral (B) views of wild-type, tli, els and coa mutant larvae at 7 dpf. The tli mutant larvae have expanded melanophores and no swim bladder. The els and coa mutant embryos have relatively normal body shape. (C–F) Plastic sections of wild-type (C), tli (D), els (E) and coa (F) mutant retinas. The stage examined was 7 dpf, except for the tli mutant that was examined at 5 dpf. In wild-type retina, three nuclear layers (RGC layer, INL, ONL) and two plexiform layers (IPL, OPL) are clearly evident (C). In the ONL, the photoreceptors differentiate and have a well-elongated OS, which is stained in dark blue (C, arrowheads). By contrast, in all three mutants, there are specific defects in the morphology of the photoreceptors. In the tli mutant, the nuclei of the ONL are observed to be normal, but the OS of the photoreceptors is specifically missing (D, the inset indicates wild-type 5 dpf photoreceptors). In the els mutant, photoreceptors are maintained, but the shape of the OS (arrowheads) is abnormal. In the coa mutant, both the ONL and OPL are absent. Furthermore, the horizontal cells are flat-shaped neurons normally located in the outer-most region of the INL, but are not observed in the coa mutant retinas. (G–J) Labeling of 8 dpf wild-type (G), tli (H), els (I) and coa (J) mutant retinas with zpr-1 antibody, which specifically labels zebrafish double-cone photoreceptors. All the nuclei are counterstained with Sytox-green (green). In wild-type retina, zpr-1-positive photoreceptors (G, red) form the ONL. In the tli mutant, zpr-1-positive photoreceptors are detected in the CMZ but almost absent in the central retina (H), suggesting that photoreceptors differentiate but degenerate in the tli mutant. In the els mutant, zpr-1-positive cells are maintained but their pattern is not regular (I). In the coa mutant, a few zpr-1-positive cells are observed in the region close to the CMZ, but they are completely absent in the central retina (J). (K–N) ERGs of 7 dpf wild-type (K), tli (L), els (M) and coa (N) mutant embryos in response to 500 ms light with an intensity of 50–60 μW/cm2. The wild-type embryo displays a typical response in ERG, which consists of a small negative a-wave and a large positive b-wave in ON-transient and a large positive d-wave in OFF-transient. However, all the three mutants show no response in ERG. |
|
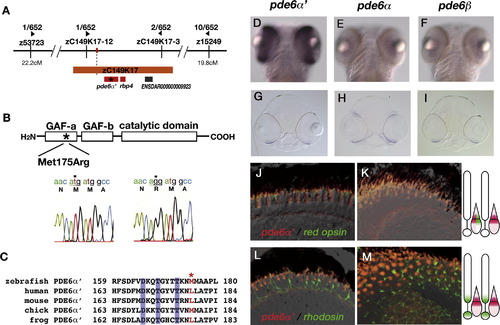
The els mutant gene encodes PDE6α′. (A) Genomic region where the els mutation locus is mapped. The els mutational locus is mapped on chromosome 12 and flanked by two polymorphic markers, zC149K17-12 and zC149K17-3. Within this flanking region, there are three candidate genes, pde6α′ (Genbank Accession No. NW_00150719), rbp4 (Genbank Accession No. NM_130920) and a novel kinesin-like gene, ENSDAR000000009923 (Zebrafish genomic database of the Sanger Institute, Ensembl ver. 30). (B) A missense mutation (175Met->Arg) occurs in the GAF-A domain of PDE6α′ in the els mutant. (C) Amino acid comparison of a part of the GAF-A domain of pde6α′ gene between zebrafish and other vertebrate species including human (Genbank Accession No. NM_006204), mouse (Genbank Accession No. NM_033614), chick (Genbank Accession No. NM_204986) and frog (Rana pipiens, Genbank Accession No: AY044176). Three amino acids indicated in blue are important for the non-catalytic binding of cGMP to PDE6α′ (Huang et al., 2004). The position of Met-175 mutated in the els mutant is indicated by an asterisk and colored red. (D–F) Ventral views of 4 dpf zebrafish embryos labeled with RNA probes for pde6α′ (D), pde6α (E) and pde6β (F). The pde6α′ mRNA is expressed exclusively in the large region of the retina. The pde6α and pde6β mRNAs are expressed in the ventral patch of the neural retina, suggesting that these two genes are expressed later than the pde6α′ gene. (G–I) Plastic sections of 4 dpf zebrafish heads labeled with RNA probes for pde6α′ (G), pde6α (H) and pde6β (I). These mRNAs are expressed in the photoreceptor layers, suggesting that these genes are expressed exclusively in photoreceptors. (J, K) Sections of 6 dpf photoreceptor layers labeled with fluorescent-conjugated RNA probes for pde6α′ (red) and red opsin (green). Central (J) and peripheral (K) retinas. (L, M) Sections of 6 dpf photoreceptor layers labeled with fluorescent-conjugated RNA probes for pde6α′ (red) and rhodopsin (green). Central (L) and peripheral (M) retinas. In both peripheral and central retinas, the expression of red opsin mRNA overlaps with that of pde6α′, whereas the expression of rhodopsin mRNA does not overlap with that of pde6α′. EXPRESSION / LABELING:
|
|
The els mutant photoreceptors show abnormalities in cell shape, cell death and opsin localization in the OS. (A, B) EM analysis of wild-type (A) and els mutant (B) photoreceptors at 6 dpf. The OS develops from the apical surface of the photoreceptors and is composed of multiple-stacked membrane discs. Beneath the OS, mitochondria accumulate to form elipsoid (e). Although the global shapes of the OS and ellipsoid are deformed in the els mutant, their fine structure seems to be normal (B). (C–E) Ultrastructures of OS (C), connecting cilia (D) and synaptic region (E) in els mutant photoreceptor, which seem normal. (F, G) TUNEL (green) of wild-type and els mutant retinas at 6 dpf. The ONL are visualized by labeling with zpr-1 antibody (red), which stains the double-cone photoreceptors. (H) Histogram of number of dying cells per eye in ONL and INL of wild-type and els mutant larvae at 6 and 9 dpf. Light blue and error bars indicate average ± standard deviation. The cell death rates in the ONL and INL are higher in the els mutant than in the wild-type, and there is a significant difference between them at 6 dpf. (I, J) Labeling of wild-type (I) and els mutant (J) photoreceptor layers with antibodies against zebrafish red opsin (red) and green opsin (green). In some of the els mutant photoreceptors, both opsins fail to be localized to the OS but spread to the basal end of photoreceptors (arrows). (K, L) Labeling of wild-type (K) and els mutant (L) photoreceptor layers with anti-blue opsin antibody (red) and zpr-1 antibody (green). In the els mutant, blue opsin is mislocalized outside of the OS (arrows). (M, N) Labeling of wild-type (M) and els mutant (N) photoreceptor layers with anti-UV opsin antibody (red) and zpr-1 antibody (green). The mislocalization of UV opsin outside of the OS is not detected in the els mutant. (O, P) Labeling of wild-type (O) and els mutant (P) photoreceptor layers with anti-rhodopsin antibody (red) and zpr-1 antibody (green). In the els mutant, rhodopsin is mislocalized outside of the OS (arrows). |
|
Both cones and rods are gradually deformed at the early stage but only cones are selectively eliminated in the els mutant. (A, B) Plastic sections of wild-type (A) and els mutant (B) retinas at 3 wpf. The ONL is thin in the els mutant retinas. (C, D) Labeling of wild-type (C) and els mutant (D) retinas with zpr-3 (red), which stains the rod OS. All the nuclei were labeled with Sytox-green (green). The rod OS is irregularly patterned in the els mutant (D). (E, F) Labeling of wild-type (E) and els mutant (F) retinas with zpr-1 (red), which stains double-cone photoreceptors (red cones/green cones). All the nuclei were labeled with Sytox-green (green). The zpr-1-positive cones are irregularly patterned and decrease in number in the els mutant (F). (G, H) OKR of 3 wpf wild-type (G) and els mutant (H) larvae under photopic condition where cone photoreceptors mainly function. Eye positions were defined by angles between the body axis and the major axis of the ellipse to which the eye shape approximates. Eye positions were plotted over time during optokinetic stimulation in one direction. The wild-type larvae showed a typical normal OKR, but there was no OKR in the els mutant, suggesting that the els mutant lacks the cone-mediated phototransduction. (I, J) OKR of 3 wpf wild-type (I) and els mutant (J) larvae under scotopic condition where rod photoreceptors mainly function. In the els mutant, a regular pattern of eye pursuit to moving stimuli and saccade was observed, suggesting that the els mutant has the rod-mediated scotopic vision. (K, L) Plastic sections of central retina of wild-type (K) and els mutant (L) larvae at 6 mpf. The morphology of the photoreceptor cell layer is abnormal in the els mutant. The layer of rod nuclei (n) becomes thick, typical cone cell morphology (c) is missing, and pigmented epithelium (p) invaginates more deeply into photoreceptor cell layer. (M, O) Labeling of central retina of wild-type (M) and els mutant (O) larvae at 6 mpf with anti-rhodopsin antibody (red). All the nuclei are counterstained with Sytox-green (green). Almost all the photoreceptors retained in the els mutant are labeled with anti-rhodopsin antibody, suggesting that most ONL cells are rods. (P, Q) Labeling of central retina of wild-type (P) and els mutant (Q) larvae at 6 mpf with zpr-1 antibody (red). All the nuclei are counterstained with Sytox-green (green). There are almost no zpr-1-positive cells in the ONL of the els mutant, suggesting that red cones and green cones are eliminated in the els adult retina. (R, S) Labeling of central retina of wild-type (R) and els mutant (S) larvae at 6 mpf with anti-blue opsin antibody (red). All the nuclei are counterstained with Sytox-green (green). There are almost no blue opsin-positive cells in the ONL of the els mutant, suggesting that blue cones are eliminated in the els adult retina. (T, U) Labeling of central retina of wild-type (T) and els mutant (U) larvae at 6 mpf with anti-UV opsin antibody (red). All the nuclei are counterstained with Sytox-green (green). There are almost no UV-opsin-positive cells in the ONL of the els mutant, suggesting that UV cones are eliminated in the els adult retina. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Ultrastructures of photoreceptors in wild-type and els mutant at adult stage. (A–B) Semi-thin sections of wild-type (A) and the els mutant (B) retinas at 12 mpf. In the outer photoreceptor layer, the regions consisting of rod OS, cone cells and rod nuclei can be distinguished. In the els mutant retina, the region where cone cells are normally located appears to be replaced with the region consisting of the OS of rods, suggesting that cone cells are specifically eliminated in the els mutant. RGCL, RGC layer. (C) OS and ellipsoid (e) of rods in wild-type. Connecting cilia are observed (arrowhead). (D) OS and ellipsoid (e) of cones in wild-type. (E) OPL in wild-type. Dense-stained structures representing synaptic ribbons (arrowhead) are observed underneath rod nuclei (n) in the OPL. (F) OS and ellipsoid (e) of rods in the els mutant. These structures are normal. (G) Connecting cilia of rods in the els mutant. (H) OPL in the els mutant. Nuclei of rods (n (rod)) and horizontal cells (n (ho)) are shown at the upper left and bottom right corners of this image, respectively. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The rods increase and the INL cells decrease in number in the adult els mutant. (A) Number of rods within 100 μm length along the line parallel to the ONL in wild-type (red bars) and els mutant (blue bars) at 6 (left) and 12 mpf (right). The averages and standard deviations are 37.6 ± 10.7 for the wild-type at 6 mpf (n = 5), 63.3 ± 5.3 for the els mutant at 6 mpf (n = 5), 46.8 ± 8.5 for the wild-type at 12 mpf (n = 4) and 47.0 ± 3.7 for the els mutant at 12 mpf (n = 4). (B) Number of INL cells within 100 μm length along the line parallel to the ONL in wild-type (red bars) and els mutant (blue bars) at 6 (left) and 12 mpf (right). The averages and standard deviations are 156.0 ± 31.0 for the wild-type at 6 mpf (n = 5), 112.2 ± 18.3 for the els mutant at 6 mpf (n = 5), 76.0 ± 19.8 for the wild-type at 12 mpf (n = 4) and 51.8 ± 3.4 for the els mutant at 12 mpf (n = 4). (C) Ratio of the number of rods to the number of INL cells in wild-type (red bars) and els mutant (blue bars) at 6 (left) and 12 mpf (right). The averages and standard deviations are 25.7 ± 12.4 for the wild-type at 6 mpf (n = 5), 58.3 ± 6.1 for the els mutant at 6 mpf (n = 5), 64.4 ± 19.6 for the wild-type at 12 mpf (n = 4) and 91.0 ± 7.4 for the els mutant at 12 mpf (n = 4). (D, E) BrdU labeling of wild-type (D) and els mutant (E) retinas at 5 wpf. Retinal plexiform layers are visualized by counterstaining with phalloidine. In the wild-type retinas, BrdU is observed not only in the CMZ (D, asterisk) but also in the cells scattered within the ONL (D, arrowheads), which appear to be rod progenitors. In the els mutant retinas, BrdU incorporation is also observed in the cells located in the INL (E, arrows), which is rarely observed in the wild-type retinas. (F) Density of BrdU-positive cells in the INL and ONL of the wild-type and els mutant retinas at 5 wpf. The number of eyes examined was four for both the wild-type and els mutant. BrdU incorporation in the INL is markedly higher in the els mutant than in the wild-type larvae (p = 1.9 x 10-5, t-test), suggesting that cell proliferation is activated in the INL of the els mutant. In the case of the ONL, BrdU incorporation is also higher in the els mutant than in the wild-type larvae (p = 0.043, t-test), suggesting that rod progenitor cells increase in number in the els mutant. |
|
Quantitative PCR analysis of rbp4 mRNA in wild-type and els mutant zebrafish embryos. Amplification of the rbp4 gene (left panels) and a control gene, β-actin (right panels), from the wild-type and the els mutant cDNA pools. The amplifications by different PCR cycles from 22 to 28 are shown from top to bottom. There is no difference in the amplification of both genes from the wild-type and the els mutant cDNA pools, suggesting that the expression level of rbp4 mRNA is normal in the homozygous els mutant embryos. |
|
Cone elimination is less severe in the peripheral retina of the els mutant than in the central retina. (A, B) Plastic sections of peripheral retina of wild-type (A) and els mutant (B) larvae at 6 mpf. (C, D) Labeling of peripheral retina of wild-type (C) and els mutant (D) larvae at 6 mpf with anti-rhodopsin antibody (red). (E, F) Labeling of peripheral retina of wild-type (E) and els mutant (F) larvae at 6 mpf with zpr-1 antibody (red). (G, H) Labeling of peripheral retina of wild-type (G) and els mutant (H) larvae at 6 mpf with anti-blue opsin antibody (red). (I, J) Labeling of peripheral retina of wild-type (I) and els mutant (J) larvae at 6 mpf with anti-UV opsin antibody (red). |
|
Pattern of zpr-1 expression in the adult els mutant retina. (A) Labeling of the els mutant retina with zpr-1 antibody (red) and anti-rhodopsin antibody (green) at 6 mpf. zpr-1-positive photoreceptors are gradually eliminated from the peripheral (left side) to the central (right side) retina. (B–C) High magnification of the image shown in (A). Peripheral (B) and central (C) regions of the retina. |
|
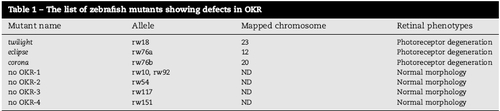
The list of zebrafish mutants showing defects in OKR |
Reprinted from Mechanisms of Development, 125(11-12), Nishiwaki, Y., Komori, A., Sagara, H., Suzuki, E., Manabe, T., Hosoya, T., Nojima, Y., Wada, H., Tanaka, H., Okamoto, H., and Masai, I., Mutation of cGMP phosphodiesterase 6alpha'-subunit gene causes progressive degeneration of cone photoreceptors in zebrafish, 932-946, Copyright (2008) with permission from Elsevier. Full text @ Mech. Dev.