- Title
-
Mypt1-mediated spatial positioning of Bmp2-producing cells is essential for liver organogenesis
- Authors
- Huang, H., Ruan, H., Aw, M.Y., Hussain, A., Guo, L., Gao, C., Qian, F., Leung, T., Song, H., Kimelman, D., Wen, Z., and Peng, J.
- Source
- Full text @ Development
|
The sq181 mutation confers a liverless phenotype. Wild-type (wt) and mutant (mu) zebrafish embryos at 3 dpf were harvested for WISH. (A,B) Lateral view using the liver-specific markers (A) prox1 and (B) lfabp. (C,D) Dorsal view using (C) a trypsin probe for the exocrine pancreas and (D) an insulin probe for the islet. (E,F) Dorsal view using (E) an ifabp probe for the intestine and (F) a foxa3 probe for the entire digestive system. Black arrows, liver; red arrows, exocrine pancreas; black arrowheads, islet; blue arrows, intestine. EXPRESSION / LABELING:
PHENOTYPE:
|
|
The sq181 mutation blocks liver bud formation but not hepatoblast specification. Wild-type (wt) and mutant (mu) zebrafish embryos at 30, 34 and 48 hpf were flat-mounted following in situ hybridization with the probes indicated on the left; dorsal view. Liver primordium, black arrows; exocrine pancreas, red arrows; islet, arrowheads. |
|
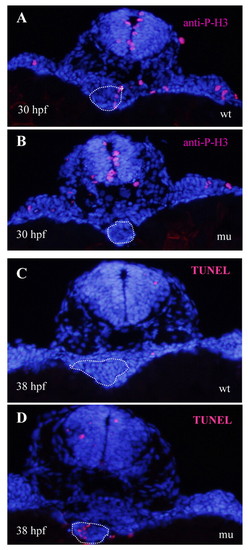
sq181 mutant hepatoblasts are impaired in proliferation and subsequently undergo cell apoptosis that leads to a liverless phenotype. (A,B) Immunostaining using an anti-P-H3 antibody on (A) wild-type (wt) and (B) mutant (mu) zebrafish embryos at 30 hpf. (C,D) TUNEL assay of cell apoptosis in (C) wild-type and (D) mypt1sq181 mutant embryos at 38 hpf. The white dotted line circles the liver primordium and the adjacent endoderm region. |
|
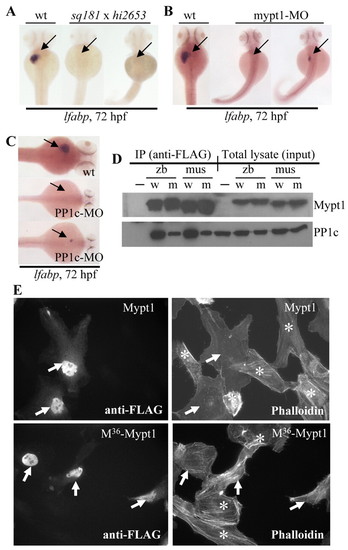
Loss-of-function of the myosin phosphatase complex causes a liverless phenotype and the mypt1sq181 mutation impairs the formation of the myosin phosphatase complex by compromising the binding of Mypt1 to PP1c. (A) Genetic complementation test between hi2653 and sq181 (sq181 x hi2653). (B,C) Both mypt1-MO and PP1c-MO morphants were either liverless or exhibited a small liver (arrow). (D) FLAG-tagged zebrafish and mouse wild-type (w) and M36-mutant (m) Mypt11-305 were co-expressed with HA-tagged mouse PP1c in COS-7 cells, and were co-immunoprecipitated using an anti-FLAG antibody. PP1c was detected with an anti-HA antibody and Mypt1 with an antibody against human MYPT1 (PPP1R12A - HUGO). Cells transfected with HA-PP1c alone were used as the negative control (-); w, wild-type Mypt11-305; m, M36-Mypt11-305; zb, zebrafish; mus, mouse. (E) Immunofluorescence microscopy of Hela cells transfected with FLAG-tagged zebrafish wild-type Mypt11-305 (Mypt1) or mutant M36-Mypt11-305 (M36-Mypt1). Cells containing Mypt1 or M36-Mypt1 were detected with an anti-FLAG antibody (arrows) (left panels). Stress fibers were stained with TRITC-labeled phalloidin (right panels). Note that cells lacking Mypt1 or M36-Mypt1 (marked with *) contained significant amounts of stress fibers, as did cells transfected with M36-Mypt11-305 (arrows, lower right panel). EXPRESSION / LABELING:
PHENOTYPE:
|
|
mypt1 is expressed in the LPM and endoderm cells. (A) Northern blot analysis of mypt1 expression in unfertilized embryos (unf), 12-hpf and 1- to 5-dpf embryos, and adult liver (adl). (B) Lateral and dorsal views of zebrafish embryos subject to WISH using the mypt1 probe. fe, foregut endoderm; hd, head region; sm, somites. (C,D) Sectioning of WISH embryos (34 hpf) with the (C) mypt1 single probe or (D) prox1 (red) and mypt1 (purple) double probe. Black arrow, liver primordium; green arrow, left LPM; yellow arrow, right LPM. nt, neural tube. |
|
The mypt1sq181 mutation alters actin assembly in LPM and endoderm cells and causes a posterior shift of the liver primordium. (A,B) Cross-sections from wild-type (WT) and mutant (mu) zebrafish embryos in the Tg(gutGFP)S584 background (green) stained with phalloidin (red). (C-F) High-magnification views of boxed regions in A (C,D) and B (E,F) showing abnormal aggregations of actin filaments (arrows) and cell organization in the mutant endoderm (C versus E) and LPM (D versus F) cells. (G,H) WISH using insulin (for islet, blue arrowheads) and hhex (for liver, arrow) probes, and F59 antibody (for somites, horizontal black lines) on mutants (mu) and wild-type (wt) embryos. |
|
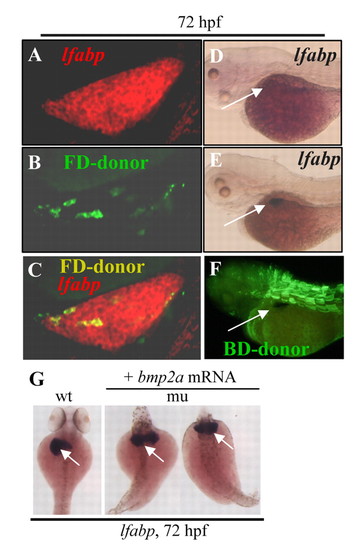
The mypt1sq181 mutation causes the liverless phenotype non-cell-autonomously and ectopic Bmp2 expression rescues liver development in the mutant. (A-C) A confocal section of the liver in a wild-type zebrafish embryo transplanted with mypt1sq181 donor cells at 72 hpf. The liver is stained with the lfabp probe (A) and the donor cells were labeled with Fluorescein-dextran (B). A and B are superimposed in C. FD-donor, Fluorescein-dextran-labeled cells. (D) mypt1-MO morphant control. (E,F) mypt1-MO morphants were transplanted with wild-type mesoderm cells (labeled with biotin-dextran). At 72 hpf, the liver was stained with the lfabp probe (E, arrow) and wild-type donor cells were visualized using the biotin tag (F). BD-donor, biotin-dextran-labeled cells. (G) mypt1sq181 mutant embryos 72 hours after injection of bmp2a mRNA, stained with the lfabp probe. wt, wild type; mu, mutant. EXPRESSION / LABELING:
|
|
The mypt1sq181 mutation alters the spatial alignment between the liver primordium and the two stripes of LPM expressing Bmp2a. (A-C) Dorsal view of zebrafish embryos subject to WISH using the bmp2a probe in wild type (wt) and mutant (mu). Somites were stained with the F59 antibody (B,C, horizontal black lines). (D,E) Dorsal view of WISH embryos using prox1 (red staining of the liver primordium, circled with a white dashed line) and bmp2a (purple) probes. (F-I) Sectioning of the prox1 and bmp2a WISH embryos at 34 hpf (F,G) and corresponding DAPI staining (H,I). Green and yellow arrows mark the left and right stripes of LPM, respectively; black arrows mark the liver primordium; red arrows mark the fin buds (fb). The white dotted line circles the liver primordium and the black dashed line shows the midline (md). (J) Schematic showing how the liver primordium at 34 hpf is sandwiched between the two bmp2a stripes in wild-type (WT) embryos, one above the primordium and one beneath, whereas in the mypt1sq181 mutant the right stripe fails to cross the midline to align with the left stripe and sandwich the liver primordium. |
|
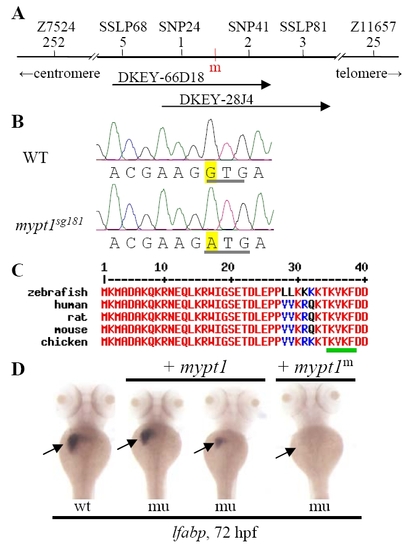
The sq181 mutation alters a conserved motif in Mypt1. (A) Integrated genetic/physical map summarizing the map-based cloning of the sq181 mutant gene. Genetic markers are shown above with their corresponding number of recombinants shown below. The mutant gene was located between SNP24 and SNP41 spanning a 45 kb genomic fragment contained in two BACs, DKEY-66D18 and DKEY-28J4. m (red), sq181 mutation locus. (B,C) Sequence traces (B) showing the G-to-A change (yellow) in the mypt1 gene in the sq181 mutant that results in a GTG codon to ATG codon change (underlined in gray) converting V36 to M in the conserved KV36xF motif in Mypt1 (underlined in green in C). (D) The liverless phenotype of the sq181 mutant (mu) was fully or partially rescued by the WT mypt1 mRNA but not by the G-to-A mutant mRNA (mypt1m). Embryos 3 days post-injection were examined with the lfabp probe. EXPRESSION / LABELING:
|
|
The mypt1sq181 mutation does not affect the polarization of LPM cells. Sections from WT (A) and mutant (mu) (B) embryos at 29 hpf were stained with a monoclonal antibody to αPKC. |
|
Bmp signaling is essential for liver development in zebrafish. (A) Immunoblot analyses of dominant-negative BmpR1a-GFP expression using an anti-GFP antibody after heat-shock treatment. Total protein lysates were prepared from heat-shocked embryos at different time intervals as indicated. (B-D) WISH using the lfabp (B), prox1 (C) or ifabp (D) probes and F59 antibody (C) on heat-shocked embryos. Embryos in B and D were heat shocked at 18-21 hpf, in C at 24 hpf. EXPRESSION / LABELING:
PHENOTYPE:
|

Unillustrated author statements EXPRESSION / LABELING:
PHENOTYPE:
|