- Title
-
Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death
- Authors
- Clay, H., Volkman, H.E., and Ramakrishnan, L.
- Source
- Full text @ Immunity
|
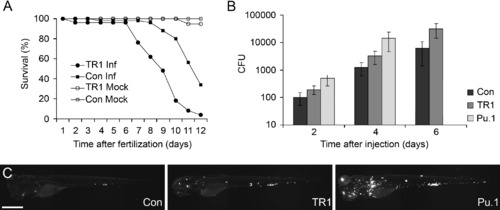
TR1 Morphant Embryos Are More Susceptible to Mycobacterial Infection (A) Control (Con) and TR1 morphant embryos were either mock injected (n = 30 each) or infected with 108 ± 11 colony forming units (CFU) of M. marinum on 1 dpf (n = 50 each). Data are plotted as the percentage of surviving embryos on each day. TR1 morphant embryos are significantly more susceptible to infection with M. marinum than controls (Hazard ratio 5.7, p < 0.0001, Kaplan Meier method with log-rank [Mantel-Cox] test). Survival was not statistically different between mock-injected TR1 and control embryos. (B) Mean bacterial loads per embryo for control (Con), TR1, and Pu.1 morphant embryos at 2, 4, and 6 days post injection (dpi) with 70 ± 13 CFU. p < 0.0001 by one-way ANOVA. Data for Pu.1 embryos are only available for 2 and 4 dpi because of mortality. Error bars represent standard deviation from the mean. All morphant bacterial loads are significantly different from one another (p < 0.05) at each individual time points (control versus TR1, control versus Pu.1, and TR1 versus Pu.1) by Student's unpaired t test. (C) Representative pictures of control, TR1, and Pu.1 morphant embryos at 4 dpi with fluorescence representing bacterial load. The scale bar represents 500 μm. PHENOTYPE:
|
|
Granulomas Form More Quickly in the Absence of TNF Signaling (A) DIC (left) and fluorescence (right) images of granuloma. Activated genes (gag3.13) in granulomas in both control and morphant embryos are shown as percentage of embryos with activation of fluorescent reporters ± the standard deviation of the mean. Scale bars represent 25 μm. (B) Three pools of ∼30 embryos were injected with 56 ± 18 CFU of gag7, and four pools of ∼30 embryos were injected with 64 ± 17 CFU of gag3.13. All groups were scored at 4 dpi for induction of gags as indicated by fluorescent activity. The average percentage of embryos with gag induction is plotted ± standard deviation of the mean. Averages for both gag7 and gag3.13 are significantly different between controls and morphants (p < 0.05, Student's unpaired t test). (C–E) 23 Control and 14 TR1 morphant embryos were injected with 89 ± 4 CFU and followed sequentially for 5 dpi and monitored for granuloma formation and size. All dark bars represent control morphant data; light bars represent TR1 morphant data. Data are plotted as average ± standard error of the mean. (C) shows the percentage of embryos with at least one granuloma over time. TR1 morphant embryos have significantly more granulomas at 3 dpi (p < 0.01) as analyzed by Fisher's exact test of a contingency table. (D) shows the average number of granulomas identified by DIC and fluorescent microscopy over time. (E) shows that TR1 morphant granulomas are significantly larger than control granulomas (p < 0.05 at 3 dpi, p < 0.0001 at 4 and 5 dpi by Student's unpaired t test, n = 14; control and 14 TR1 granulomas at 3 dpi, 75 control and 53 TR1 granulomas at 4 dpi, and 113 control and 72 granulomas at 5 dpi). The y axis represents granuloma diameter in μm. (F and G) Pools of 20 embryos were injected with 27 ± 6 wild-type (WT) and 51 ± 13 ΔRD1 M. marinum and assayed by microscopy at 4 dpi for granuloma formation. (F) shows representative images of control and TR1 morphant embryos. Arrowheads indicate granulomas. The scale bar represents 200 μm. As shown in (G), the average percentage of embryos with granulomas is plotted ± standard deviation of the mean over four biological replicates. The percentage of embryos with granulomas is significantly higher when injected with WT (p < 0.01 for Con; WT versus Con; ΔRD1 and p < 0.001 for TR1; WT versus TR1; ΔRD1 by Student's unpaired t test). |
|
Growth of Erp Mutant Bacteria Is Partially Restored in TR1 Morphant Embryos (A) Representative fluorescence images for control (Con) and TR1 morphant fish infected with 55 ± 7 CFU of Erp mutant (Erp) M. marinum at 4 dpi. The scale bar represents 500 μm. (B–D) A total of 50 individual macrophages were scored for multiplicity of infection (MOI) as either containing (B) 10 or less bacteria or (C) more than 10 bacteria at 3 dpi during infection with 55 ± 7 CFU of Erp mutant bacteria. The scale bar represents 25 μm. As shown in (D), the percentage of macrophages (out of 50) scored as either having more than ten, or ten or less bacteria per macrophage ± standard deviation of the mean in TR1 morphant versus control embryos. TR1 morphants had a significantly greater percentage of macrophages with more than ten bacteria as compared to controls (p < 0.05, Student's unpaired t test). |
|
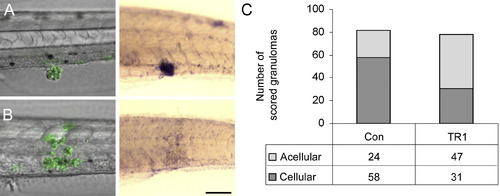
TR1 Morphant Embryos Have Significantly More Acellular Granulomas than Controls (A and B) Differential interference contrast overlay with fluorescence (left) of infected embryos then labeled with in situ hybridization for the macrophage marker fms (right). Green indicates M. marinum in fluorescent overlays. Purple staining of hybridized embryos indicates expression of the macrophage marker fms. (A) shows an example of a cellular granuloma in a control embryo before and after in situ hybridization indicating bacteria within aggregated macrophages. (B) shows an acellular granuloma example in a TR1 morphant embryo indicating bacteria found primarily outside of macrophages. (C) The number of granulomas scored as either cellular or acellular in TR1 morphant versus control embryos. TR1 morphant granulomas were twice as likely to contain one-third or fewer fms-positive cells than to WT granulomas (p < 0.0001) as analyzed by Fisher's exact test of a contingency table. Embryos were 7 dpf and infected with 267 ± 28 CFU. |
|
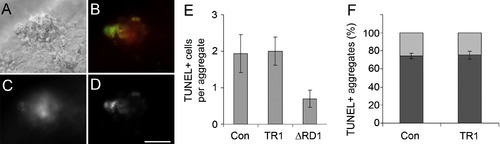
TNF Signaling Does Not Influence the Rate of Apoptosis of Infected Cells DIC and fluorescent imaging of a granuloma are shown in (A) and (B)–(D), respectively. (B) shows colocalization of TNF expression (red) and TUNEL-labeled double-strand DNA breaks (green). Individual fluorescence channels for (C) TNF expression and (D) TUNEL-labeling are shown. The scale bar represents 50 μm. (E) shows the number of TUNEL-positive cells per granuloma ± standard deviation of the mean. The average number of TUNEL-positive cells within granulomas in 4 dpi embryos is unchanged between control (n = 16 granulomas) and TR1 morphant embryos (n = 20). Infection of control embryos with RD1-deficient bacteria (ΔRD1) is used as a control (n = 20). The number of TUNEL-positive cells in ΔRD1 granulomas is significantly less than control and TR1 morphant granulomas with wild-type bacteria (p < 0.05 by Student's unpaired t test for both comparisons). Granulomas were selected to be between 40 and 50 μm in diameter to normalize for total cell number. (F) shows the percentage of TUNEL-positive granulomas between control and TR1 morphant 4 dpi embryos ± standard error of the mean. Three separate experiments of pools of 20–40 granulomas were scored per condition and plotted as number of granulomas with TUNEL-positive cells over total granuloma number. Error bars represent standard error of the mean. PHENOTYPE:
|
|
TR1 Morphant Embryos Are More Likely to Have Extracellular Bacteria as Indicated by Cording DIC (A) and fluorescence (B–E) imaging of bacteria in vivo. As shown in (A) and (B), extracellular bacteria in Pu.1 morphant embryos without macrophages display the cording phenotype. The scale bar represents 25 μm. As shown in (C) and (D), flattened 3D Z stacks of control (C) and TR1 morphant (D) granulomas demonstrate cording in TR1 morphant embryos. Scale bars represent 50 μm. (E) shows cording after what appears to be breakdown of an individual infected macrophage in infected TR1 morphant embryo. The scale bar represents 25 μm. As shown in (F), four pools of 30 embryos were injected with 18 ± 5 CFU and scored daily for the presence of extracellular bacteria as indicated by cording and plotted as the percentage of embryos with cording ± the standard deviation. TR1 morphant embryos had significantly higher percentages of embryos with cording at all time points by Student's unpaired t test (p < 0.05 for 3 and 4 dpi, p < 0.01 for 5 dpi). |
|
Increased Cording Seen in the Absence of TNF Signaling Is Not Dependent on the RD1 Virulence Locus or Granuloma Formation (A) Control (n = 23) and TR1 morphant (n = 9) embryos were infected with 353 ± 10 ΔRD1 bacteria and scored daily for the presence of extracellular bacteria as indicated by cording and plotted as the percentage of embryos with cording. A significantly greater percentage of TR1 morphant embryos display cording versus control embryos at 5 dpi (p < 0.01) and 6 dpi (p < 0.05) as analyzed by Fisher's exact test of a contingency table. (B) and (C) show examples of cording found during infection of TR1 morphant embryos infected with ΔRD1 at 6 dpi. The scale bar represents 25 μm. PHENOTYPE:
|
|
Morpholino targeting of the TNF Receptor 1 (TR1) leads to complete splice blocking of transcript for 4 days. |
|
Inducible nitric oxide synthase (iNOS) antibody staining is similar in infected control and TR1 morphant embryos. |
|
Macrophage death can lead to dissemination of infection. |
Reprinted from Immunity, 29(2), Clay, H., Volkman, H.E., and Ramakrishnan, L., Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death, 283-294, Copyright (2008) with permission from Elsevier. Full text @ Immunity