- Title
-
Sphingosine-1-phosphate receptors regulate individual cell behaviours underlying the directed migration of prechordal plate progenitor cells during zebrafish gastrulation
- Authors
- Kai, M., Heisenberg, C.P., and Tada, M.
- Source
- Full text @ Development
|
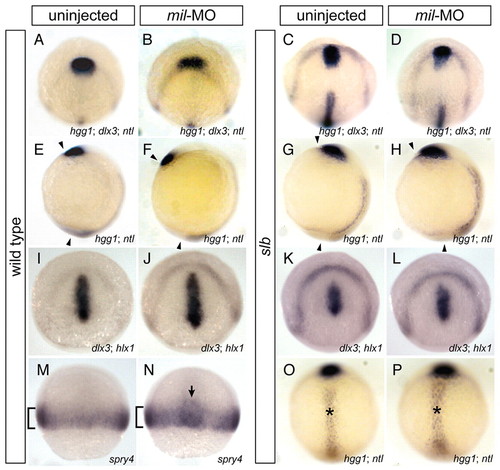
Abrogation of Mil function facilitates anterior migration of the presumptive prechordal plate. In situ hybridisation (A-H,O,P) of dlx3 (anterior border of the neural plate), hgg1 (anterior prechordal plate) and ntl (notochord) at tailbud stage, (I-L) of dlx3 and hlx1 (posterior prechordal plate) at tailbud stage, and (M,N) of spry4 (presumptive mesoderm) at 60% epiboly. (A-D) Dorso-animal views, (E-H) lateral views, (I-L) dorso-animal views and (M-P) dorsal views of wild-type (WT) embryos (A,E,I,M), WT embryos injected with 4.3 ng mil-MO (B,F,J,N), slb embryos (C,G,K,O) and slb embryos injected with 4.3 ng mil-MO (D,H,L,P). In mil-MO-injected embryos, anterior migration of the presumptive prechordal plate was promoted (B,F), as compared to WT (A,E), with regards to relative positions of the prechordal plate in relation to the anterior border of the neural plate (A-D), and to angles between the prechordal plate and the tailbud, indicated by arrowheads (E-H). Injection of mil-MO rescued the reduced migration of the anterior prechordal plate in the slb embryo (C,D,G,H), but did not alter the wider notochord (O,P, indicated by asterisks). By contrast, the altered position of the posterior prechordal plate correlated with that of the anterior prechordal plate (K,L). (M,N) The enhancement of migration was not observed in the lateral mesoderm (brackets), but was specific to the prospective prechordal plate (arrow). |
|
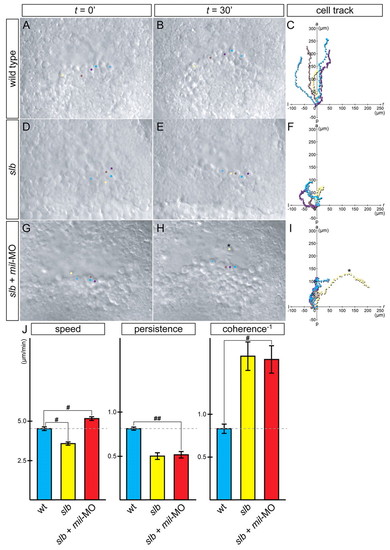
slb prechordal plate progenitors migrate individually with increased motility and reduced cohesion when Mil function is compromised. (A-I) Time-lapse analysis and tracking of cell movement in migrating prechordal plate progenitors of WT embryos (A-C), slb embryos (D-F) and slb embryos injected with 4.3 ng mil-MO (G-I). Five cells were randomly chosen from each embryo, highlighted in different colours and tracked by merging each starting point to 0 (C,F,I). Twenty-five cells in total were accumulated from five independent experiments, to analyze for speed, persistence and coherence (J). In slb embryos, cell motility was significantly reduced (D-F). In slb embryos injected with mil-MO, the cells showed increased motility, and the cluster of cells appeared to be less coherent (G-I); the cells occasionally popped out from the cluster and travelled individually (H,I, asterisk). Such cell behaviour was never observed in WT embryos. (J) Summary of analysis of parameters: speed, persistence and coherence. Statistically significant differences are indicated (#P<0.05, ##P<0.005). PHENOTYPE:
|
|
mil is required for the stabilisation of cellular processes towards the direction of migration. The orientation and length of lamellipodia-like cellular processes of leading edge cells in a WT embryo (A), slb embryo (B) and slb embryo injected with 4.3 ng mil-MO (C). The morphology of a cell from each group is highlighted in the insets. The length of the long axis of cells and their orientation with respect to the direction of movement (y-axis) were calculated and plotted, and the angle from the y-axis was calculated as cosine. Statistically significant difference is indicated (##P<0.005). PHENOTYPE:
|
|
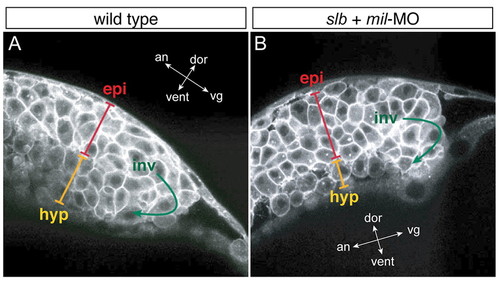
slb cells with compromised Mil function undergo involution differently to WT cells. Multi-photon confocal analysis of involution cell behaviour through the shield region. Lateral views of WT embryo (A) and slb embryo injected with 4.3 ng mil-MO (B) at 60% epiboly. In the hypoblast layer of the slb embryo injected with mil-MO, cells are internalised as one- or two-cell thick layers, occasionally with space in between the cells, whereas in the WT embryo hypoblast cells are internalised as approximately three-cell thick layers throughout and are tightly packed. epi, epiblast layer; inv, involution; hyp, hypoblast layer. The orientation of embryos is shown as indicated along the animal (an)-vegetal (vg) axis and the dorsal (dor)-ventral (vent) axis. PHENOTYPE:
|
|
Edg1 acts antagonistically against Mil in the directed migration of prechordal plate progenitor cells. (A-F) In situ hybridisation of dlx, hgg1 and ntl of WT embryos injected with 6.2 ng edg1-MO alone (A,D) or co-injected with 4.3 ng mil-MO (B,E) at tailbud stage, and of slb embryos injected with 6.2 ng edg1-MO (C,F). (G-I) In situ hybridisation of dlx3 and hlx1 of WT embryos injected with edg1-MO alone (G) or co-injected with mil-MO (H) at tailbud stage, and of slb embryos injected with edg1-MO (I). (A-C,G-I) Dorso-animal views; (D-F) lateral views. edg1-MO injection reduced anterior migration of the anterior (A,D) and posterior (G) prechordal plate, compared with WT (see Fig. 1A,E,I), and enhanced the slb prechordal plate phenotype (C,F,I) (see Fig. 1C,G,K). Co-injection of edg1-MO and mil-MO cancelled out the effect of one another and often gave rise to an embryo reminiscent to WT (B,E,H). |
|
Mil negatively regulates Pdgf-induced process formation. (A,B) WT embryos were injected with 100 pg cyclops RNA alone (A) or together with 150 pg mil RNA (B) at the one-cell stage, and were dissociated at sphere stage. The dissociated cells were then maintained on fibronectin-coated dishes and treated with 50 ng/ml of Pdgf 30 minutes prior to observation. The frequency of Pdgf-induced lamellipodia-like cellular processes decreased when mil RNA was overexpressed (22.2%), compared with those of WT cells (64.7%, B). Similarly, the length of processes in cells overexpressing mil RNA was reduced (27.3±4.8 μm, n=18) compared with those of WT cells (40.3±9.9 μm, n=17). (C-F) WT embryos were injected with 100 pg cyclops RNA along with RNAs coding for PH-GFP (125 pg) and membrane-RFP (125 pg) in the absence (C,E) or presence (D,F) of 150 pg mil RNA, and were dissociated for culture as described above. In controls (C,E), the cells responded to Pdgf stimulation and recruited PH-GFP to the plasma membrane. In cells overexpressing mil RNA, PH-GFP was dispersed in the cytoplasm (D), compared with the referential signal on the membrane (F). Analysis of the relative intensity of fluorescent signals along the lines is shown below each image (C-F). |
|
mil requires PI3K to modulate cell motility and process formation but not directionality. (A,B) The orientation and length of cellular processes of WT embryos injected with 250 pg dn-PI3K alone (A) or with 4.3 ng mil-MO (B). The morphology of the cells is highlighted in the insets. The length of the long axis of cells and their orientation with respect to the direction of movement (y-axis) were calculated and plotted, the angle from the y-axis was calculated as cosine. (C) Summary of analysis of parameters: speed, persistence and coherence. Statistically significant differences are indicated (#P<0.05 and ##P<0.005). |
|
Abrogation of Mil and Edg1 function modulates movements of the presumptive hypothalamus. (A-D) Uninjected WT embryo (A) and WT embryos injected with 4.3 ng mil-MO (B), 6.2 ng edg1-MO (C) or 4.3 ng mil-MO + 6.2 ng edg1-MO (D). The embryos were fixed at tailbud stage for in situ hybridisation with the markers dlx3 (for the anterior edge of the neural plate), pax2.1 (for the prospective mid-hind brain boundary) and nkx2.1 (for the presumptive hypothalamus). The position of the presumptive hypothalamus is highlighted by the dotted lines. |
|
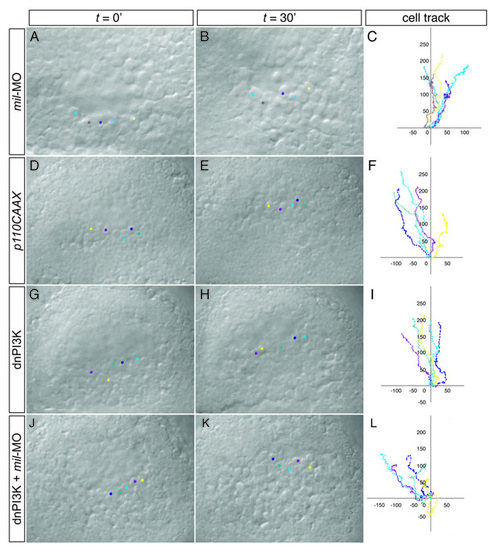
Time-lapse analysis and tracking of cell movement in migrating prechordal plate progenitors. (A-L) WT embryos injected with 4.3 ng mil-MO (A-C), 125 pg p100CAAX (ca-PI3K) RNA (D-F), 250 pg dn-PI3K RNA (G-I) or 250 pg dn-PI3K RNA together with 4.3 ng mil-MO (J-L). PHENOTYPE:
|