- Title
-
A dynamic fate map of the forebrain shows how vertebrate eyes form and explains two causes of cyclopia
- Authors
- England, S.J., Blanchard, G.B., Mahadevan, L., and Adams, R.J.
- Source
- Full text @ Development
|
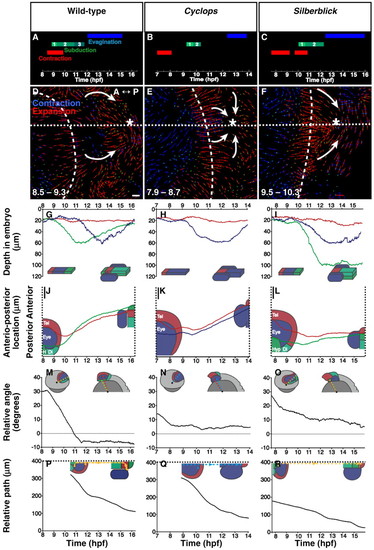
Eye-field morphogenesis during forebrain neurulation. (A-D,I-L) Projections of GFP-labelled nuclei (also see Movie 1 in the supplementary material). Dorsal projection, anterior is left. (E-H,M-P) 3D projections of tracked cells (also see Movies 2 and 3 in the supplementary material). Frontal projection, dorsal is up. (A,E) Forebrain regions prior to neurulation (midline, broken line). (B,F) Anterior neural plate contracts posteriorly towards the hypothalamic tip, where neural keel formation commences (asterisk). (C,G) Subducting hypothalamus moves anteriorly beneath the medial eye field. Convergence narrows the neural plate, which begins to fold lateral to medial, forming a neuropore (broken line). Posterior eye field moves anteriorly. (D,H) Hypothalamus emerges anterior and ventral, eye field remains contiguous across midline. (I,M) Eye evagination begins with cells moving into vesicles as posterior-lateral optic stalk and diencephalon converge and move anteriorwards. (J,N) Medial eye field continues to move into eye vesicles. (K,O) Lateral telencephalon meets at the midline to close the neuropore; anterior eye-field splitting is complete (arrows). Posterior optic stalk arrives beneath anterior stalk precursors. (L,P) Eye tissue now within eye vesicles. hpf, hours post fertilisation. Asterisks indicate anterior tip of neural keel formation (hypothalamus). Arrows indicate the direction of movement of the cells. Time (bottom left-hand corner) in hpf. Colours are indicated in A. A, anterior; P, posterior. Scale bars: 25 μm. |
|
Quantitative comparison of forebrain morphogenesis in wild type, cyc morphant and slb. (A,D,G,J,M,P) Wild type. (B,E,H,K,N,Q) cyc morphant. (C,F,J,L,O,R) slb. Dotted line, midline; dashed line, neural-plate border. (A-C) Timing of principal morphogenetic phases. Keel subduction is subdivided into: 1, moving deep; 2, moving forwards and deep; and 3, moving forwards. (D-F) Neural-plate contraction shown by a measure of tissue deformation. Dorsal view, anterior to left, as indicated. The orientations of deformation vectors show the principal directions and their length proportional to magnitude. Neural and non-neural ectoderm is distinct. In wildtype and cyc morphant embryos, keel initiation (asterisk) is coincident with the focal point of neural-plate contraction (arrows), but in slb these two behaviours are dissociated. (G-I) Depth within the embryo of medial tissue during keel formation. Hypothalamus undergoes subduction in wild type (G) and slb (I); the medial eye field subducts in cyc morphant (H). Telencephalon remains shallow in all cases. (J-L) Locations of the subducting tissues relative to the telencephalon, shown by location along the anterior-posterior axis. (M-O) The same data is plotted as angular positions measured around the great circle along the midline, relative to an arbitrary anterior reference point. (P-R) Rate of eye evagination was measured from the movement of posterior eye-field cells that remain connected to the neural tube, after evagination, relative to their anterior counterparts. These are future optic-stalk cells in wild type (P) and slb (R), and the residual band of retina in cyc morphant (Q). Positions were followed along the path of movement rather than the straight-line distance. Reduced anterior-posterior reorganisation in slb is evident. hpf, hours post fertilisation. (D,F) Time (bottom left-hand corner) in hpf. Colours for A-C,G-R are indicated in A; and for D-F in D. A, anterior; P, posterior. Scale bars: 25 μm. PHENOTYPE:
|
|
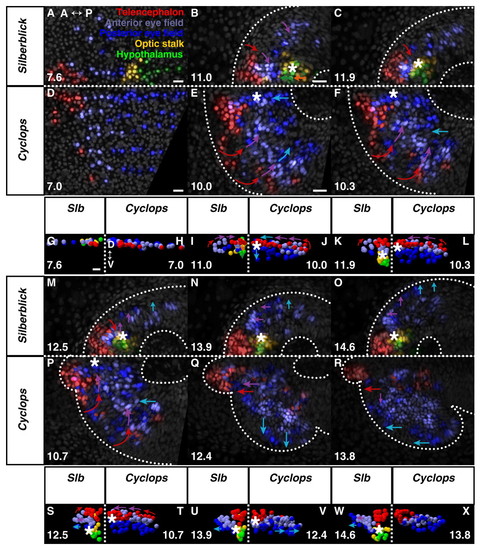
Eye morphogenesis in cyc morphant and slb. (A-F,M-R) Dorsal projections, anterior to left; (G-L,S-X) frontal projections, dorsal up. slb (A,G) and cyc morphant (D,H) forebrain regions prior to neurulation. Anterior limit of keel formation, as with wild-type, begins with hypothalamus in slb (B) but is far anterior within the anterior eye field in cyc morphant (E). (C,F) Keel movement is attenuated in both cyc morphant and slb, relative to wild-type, never emerging anterior to the telencephalon. (I,K) Anterior tissue is displaced progressively deep in slb. (J,L) Medial eye field occupies the ventral midline in cyc morphant. (M-O) Retarded medial eye-field reorganisation and evagination in slb. (S,U,W) Deep keel persists in slb. (P,Q,T,V) Normal medial convergence of telencephalon and anterior eye field in cyc morphant. (R,X) Subducted eye field remains medial within keel after evagination. Square dotted line, midline. Asterisk, anterior tip of neural keel. Arrows indicate the direction of movement of the cells. Time (bottom left- or right-hand corner) in hpf. Colours are indicated in A. See Movies 7 (slb), 8 (cyc morphant), 9 and 10 in the supplementary material. A, anterior; P, posterior; D, dorsal; V, ventral; hpf, hours post fertilisation. Scale bars, 25 μm. PHENOTYPE:
|
|
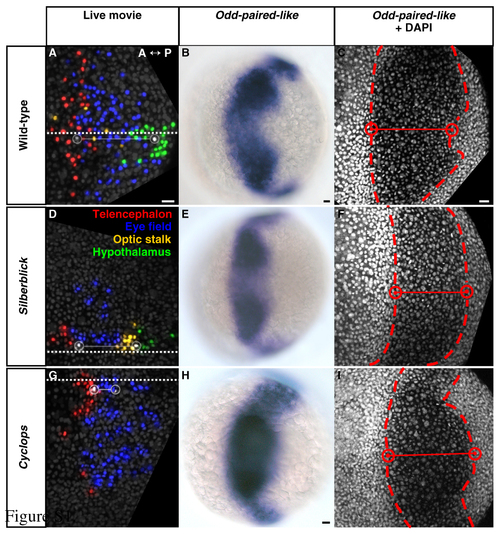
Initiation of keel formation relative to eye-field location in wild-type, silberblick and cyclops embryos. (A-C) Wild type. (D-F) slb. (G-I) cyc morphant. (A-I) Dorsal views, anterior to left. (A,D,G) Forebrain regions, defined by cell tracking, at 80% epiboly (8.4 hpf). White cells and open circles - medial anterior eye (left) and initiation of keel formation (right). Adjoining solid line - intervening distance along the midline (square dotted line). Keel formation begins with hypothalamus in wild-type (A) and slb (D), and with anterior eye field in cyc morphant (G). For corresponding angular distances subtended at the embryonic centroid see Table S1. (B,E,H) Odd-paired-like (opl) gene expression, as determined using standard procedures, defines the eye field at 80% epiboly (8.4 hpf). Hypothalamus occupies the posterior-medial notch in wild type (B) and slb (E), consistent with the tracked data in A and D. However, eye tissue presides in the posterior-medial notch in cyc morphant (H). (C,F,I, Table S1) DAPI nuclear counter-staining, defining the medial boundaries (red cells and open circles) of the opl-expressing eye field (dashed line) at 80% epiboly (8.4 hpf), confirms this. hpf, hours post fertilisation. Scale bars: 25 μm. EXPRESSION / LABELING:
|
|
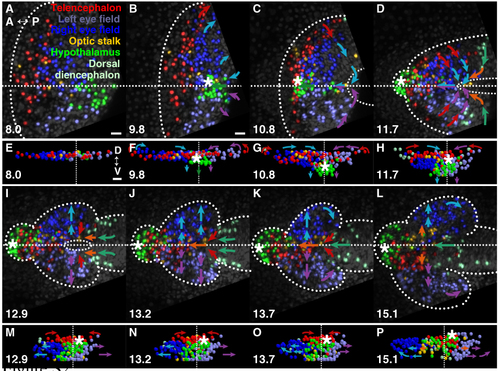
The resolution of bilateral eyes during forebrain neurulation. (A-D,I-L) Projection images of GFP-labelled nuclei. (E-H,M-P) 3D projections of tracked cells. Arrows indicate the direction of major tissue movement. Dotted line, neural boundary; dashed lines, dorsal midline, neuropore. (A-D,I-L) Dorsal views, anterior is left; (E-H, M-P) frontal views, dorsal is up. (A,E) Forebrain regions prior to neurulation. Cells of the left and right eyes are intermixed at the midline. Left and right eye-field cells remain intermixed throughout the period of posterior contraction of the anterior neural plate (B,F) and throughout subduction of the hypothalamus (asterisk) beneath the medial eye field (C,D,G,H). (I, M) Sorting of eye tissue into the appropriate vesicles begins with the movement of cells away from the midline during evagination, (J-L, N-P) and continues coincident with the closure by the telencephalon of the neuropore, and the convergence and anterior-ward movement of the dorsal diencephalon. hpf, hours post fertilisation. Scale bars: 25 μm. |