- Title
-
Expression of zebrafish pax6b in pancreas is regulated by two enhancers containing highly conserved cis-elements bound by PDX1, PBX and PREP factors
- Authors
- Delporte, F.M., Pasque, V., Devos, N., Manfroid, I., Voz, M.L., Motte, P., Biemar, F., Martial, J.A., and Peers, B.
- Source
- Full text @ BMC Dev. Biol.
|
Zebrafish pax6b is expressed in endocrine pancreatic cells in contrast to the zebrafish pax6a gene. (A, B) At 24 hours of development, both pax6a and pax6b mRNAs are detected by in situ hybridization in the eye and in the telencephalon but only pax6b is expressed in the pancreas. (C-D′) At 48 hours post fertilization (hpf), pax6a and pax6b are expressed in overlapping areas, such as the ocular stuctures (retina and lens), the ventral telencephalon and the diencephalon. pax6a is detected more strongly in the hindbrain whereas only pax6b is expressed in the pancreatic endocrine islet. (E) pax6b colocalizes with neurod in the differentiating endocrine cells by 30 hpf. (F) No pancreatic expression of pax6a is detected at 48 hpf, as demonstrated by double WISH with pax6a and pdx1. (G-R) Double fluorescent in situ hybridizations on 30 hpf zebrafish embryos show pax6b colocalization with the four major hormones glucagon, insulin, somatostatin and ghrelin. Abbreviations: e, eye; gcg, glucagon; ghrl, ghrelin; hb, hindbrain; ins, insulin; p, pancreas; sst, somatostatin; tc, telencephalon. Embryos in figures A-R are presented in a ventral view, anterior to the left. Embryos in C′-D′ are presented in a lateral view, anterior to the left. |
|
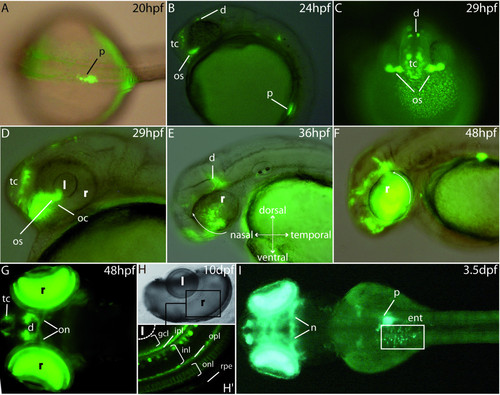
GFP expression pattern in the stable P0-pax6b:gfp transgenic zebrafish. (A) Dorsal view of a 20 hpf transgenic embryo showing GFP expression in the developing pancreas. (B, C) Lateral and frontal views at 24 and 29 hpf showing GFP expression in the ventral telencephalon, the dorsal diencephalon and the optic stalks. (D, E, F) Lateral views of an eye of 29 hpf, 36 hpf and 48 hpf transgenic embryos. GFP expression starts at about 29 hpf in a small cluster of cells in the ventronasal retina, near the optic stalks, and subsequently spreads to the entire retina (white arrows). (G) After 2 days of development, expression is observed in the neuroretina, the optic nerves as well as the ventral telencephalon and some cells of the diencephalon. (H, H′) At 10 dpf, expression is clearly detected in several layers of the retina, mainly the inner plexiform layer, the inner nuclear layer, the outer plexiform layer and the outer nuclear layer. (I) Finally, from 3.5 dpf, the transgenes are expressed in the enteroendocrine cells of the intestine and in some neurones of the mesencephalon. Abbreviations: d, diencephalon; e, eye; ent, enteroendocrine cells; gcl, ganglion cell layer; hb, hindbrain; inl, inner nuclear layer, ipl, inner plexiform layer; l, lens; mb, midbrain; nt, neural tube; oc, optic choroid; on, optic nerves; onl, outer nuclear layer; opl, outer plexiform layer; os, optic stalks; p, pancreas; pcl, photoreceptor cell layer; r, retina; rpe, retinal pigmented epithelium; tc, telencephalon. A, G and I are dorsal views (anterior to the left); B, D, E and F are lateral views (anterior to the left); H, H′ are cross sections in the eye of a 10 dpf embryo. EXPRESSION / LABELING:
|
|
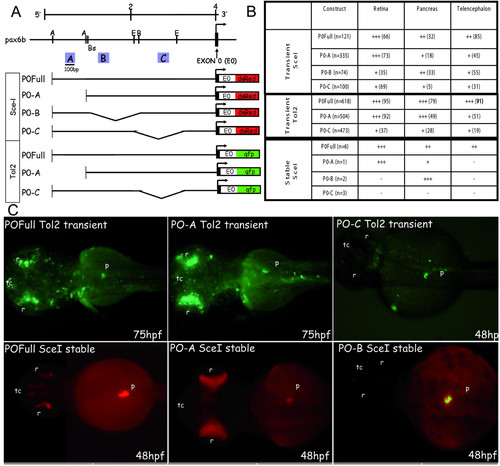
GFP/DSRED expression in transient and stable zebrafish transgenic embryos obtained with deletion constructs of pax6b P0 promoter. (A) Restriction map of the zebrafish pax6b genomic locus. The arrow indicates the transcriptional start site at exon 0 (E0, marked in black). Promoter deletion constructs were made by enzymatic digestion at the sites indicated. A, B and C conserved regions of approximately 100-bp long are indicated by blue boxes. (B) These DNA constructs were injected into zebrafish embryos and DSRED (Sce-I transgenesis) or GFP (Tol2 transgenesis) transient expression was analysed at 24, 48 and 75 hours of development. The percentage of embryos expressing DSRED/GFP in the retina, the pancreas and the telencephalon versus the total number of embryos expressing DSRED/GFP (n) is indicated in brackets; the number of DSRED/GFP-expressing cells in each tissue (from +++ to +) is shown in the table. For the stable transgenic lines, n is the number of different transgenic founders obtained, and the level of DSRED expression is indicated (from +++ to -). (C) Transient GFP expression in 75 and 48 hpf embryos injected with the full PO (FullP0), the A-deleted (P0-A) and the C-deleted (PO-C) constructs using Tol2-mediated transgenesis. DSRED expression in stable transgenic embryos at 48 hpf harboring the full P0 construct, the A-deleted construct or the B-deleted construct after Sce-I transgenesis. Abbreviations: A, AccI; B, BclI; Bs, Bst11071I; E, EcoNI; p, pancreas; r, retina; tc, telencephalon. All the embryos are presented in dorsal views with anterior on the left. EXPRESSION / LABELING:
|
|
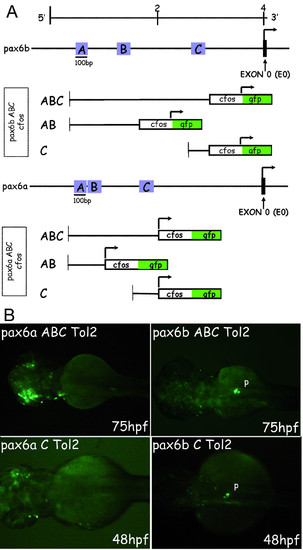
GFP expression in transient zebrafish transgenic embryos obtained with constructs carrying pax6b and pax6a AB and/or C regions fused to the cfos heterologous promoter. (A) Restriction map of the zebrafish pax6b and pax6a genomic locus. The arrow indicates the transcriptional start site at the exon 0 (E0, marked in black). For the constructs, the different regions to test were fused to the cfos heterologous promoter. The arrow indicates the transcriptional start site at the cfos. Fusion constructs (AB-cfos and C-cfos) were made by PCR using specific primers (see materials and methods for sequences). These DNA constructs were injected into zebrafish embryos using Tol2 mediated transgenesis and GFP transient expression was analysed at 24, 48 and 75 hours of development. (B) Transient GFP expression in 75 and 48 hpf embryos injected with the full ABC-cfos and the C-cfos constructs. All the embryos are presented in dorsal views with anterior on the left. Abbreviations: p, pancreas. |
|
PBX and PREP homeoproteins bind to the P0-pax6b PA2 element and with PDX1 to the P0-pax6b PA1 element. (A) EMSAs were performed on PA1 probe of pax6b (PA1b) using nuclear extract of pancreatic TU6 cells. 10 ng of unlabeled oligonucleotides PA1b, UE-A or mutated UE-A (sequences depicted below the lanes) were added as competitor, as indicated above the lanes. Position of complexes T′, T, L and S are indicated by the arrows. (B) EMSAs were performed on PA2 probe of pax6b (PA2b) using nuclear extract of pancreatic TU6 cells. 10 ng of unlabeled oligonucleotides PA2b (sequence depicted below the lanes), PA1b, UE-A or mutated UE-A were added as competitor. Position of complexes L and S are indicated by the arrows. Abbreviations: PBX1a and PREP1, in vitro-translated PBX1a and PREP1 proteins; PDX1, recombinant PDX1; PDX1 Ab, PDX1 antiserum; PBXL. Ab, antiserum recognizing specifically the long PBX isoforms (PBX1a, PBX2, and PBX3a) (Santa Cruz Biotechnology); PREP1 Ab, PREP1 antibody; nsp., nonspecific complex, Ret. lysate, reticulocyte lysate. |
|
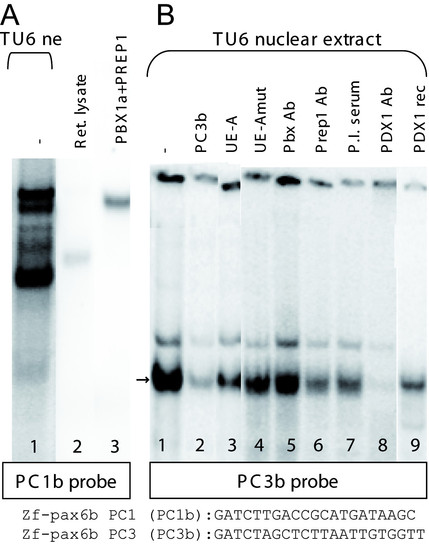
PBX-PREP dimers bind to the P0-pax6b PC1 element and PDX1 binds to PC3b as a monomer. (A) EMSAs were performed on PC1 probe of pax6b (PC1b) using nuclear extract of pancreatic TU6 cells. (B) EMSAs were performed on PC3 probe of pax6b (PC3b) using nuclear extract of pancreatic TU6 cells. 10 ng of unlabeled oligonucleotides PC3b, UE-A and mutated UE-A were added as competitor. Abbreviations: PBX1a and PREP1, recombinant PBX1a and PREP1 proteins produced in vitro in a reticulocyte extract; PDX1 Ab, PDX1 antiserum; PBXL. Ab, antiserum recognizing specifically the long PBX isoforms (PBX1a, PBX2, and PBX3a) (Santa Cruz Biotechnology); PREP1 Ab, PREP1 antibody PBX1a and PREP1; P.I. serum, pre-immune serum. |
|
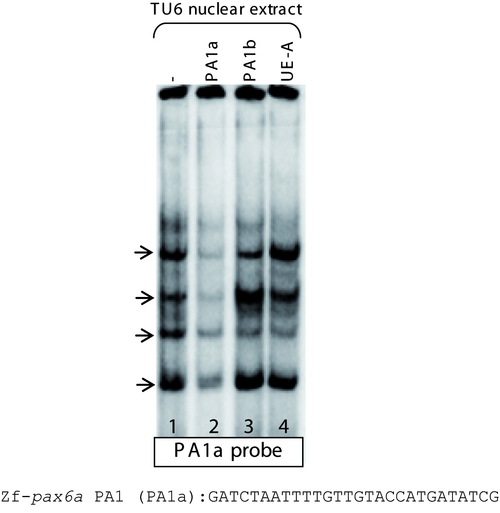
No PBX-PREP-PDX1 complex is observed on homologous pax6a PA1 sequence. When we tested the homologous pax6a PA1 sequence (named PA1a), incubation with nuclear extract led to the formation of four major complexes (arrows, lane 1) which were displaced by adding a 100-fold molar excess of unlabeled PA1a (lane 2). Addition of unlabelled PA1b and somatostatin element UE-A, which bind PBX-PREP-PDX1 and PBX-PREP complex, respectively, do not displace the binding on the probe (lanes 3 and 4). This indicates that the proteins in the pancreatic extract which recognize the two cis-elements PA1b and UE-A do not recognize the homologous pax6a element. |
|
Unlabeled PA1 of pax6a does not compete with PA1 of pax6b. To further demonstrate that PDX1 is unable to bind to PA1a, this sequence was used as competitor on PA1b probe. No competition was observed, suggesting that PDX1 binds specifically PA1 of pax6b and not PA1a. |
|
PDX1 binds to PC3b and not to PC3a. EMSAs were performed on PC3 probes of pax6a (PC3a) and pax6b (PC3b) using nuclear extract of pancreatic TU6 cells. 10 ng of unlabeled oligonucleotides PC3a (lanes 2, 6), and PC3b (lanes 3, 7) were added as competitors. Abbreviations: PBX1a and PREP1, recombinant PBX1a and PREP1 proteins produced in vitro in a reticulocyte extract; PDX1 rec, PDX1 recombinant. |