- Title
-
The multidomain protein Brpf1 binds histones and is required for Hox gene expression and segmental identity
- Authors
- Laue, K., Daujat, S., Crump, J.G., Plaster, N., Roehl, H.H., Tübingen 2000 Screen Consortium, Kimmel, C.B., Schneider, R., and Hammerschmidt, M.
- Source
- Full text @ Development
|
Zebrafish brpf1 mutants display anterior shifts in pharyngeal arch identities. Genotypes of fish are indicated in upper right corners (WT, wild type; -/-, homozygous brpf1 mutant; MO, brpf1 morphant), stages in lower right corners. (A,B) Lateral views of live larvae. (C-L) Cartilaginous elements of visceral skeleton stained with Alcian Blue (AB). (C-I) Ventral views; neurocranium has been removed. Numbers of pharyngeal arches are indicated (1-7). Arrowheads (D,E,G) point to absent basihyal (bh) of mutant arch 2. In addition, arches 3 and 4 of the brpf1 mutant lack hypobranchials (hb) (G, asterisks), intermediate elements that (in wild-type larvae) are characteristic for arches 3-7, but absent in arches 1 and 2 (C,F). Furthermore, the distal ends of the mutant ceratobranchials (cb) (I; 3,4) have acquired the shape and organization of the ceratohyal (ch) of the second arch of wild-type larvae (H; 2). (J-L) Lateral views of arches 1 and 2. Arrows in K point to joints between ventral and dorsal elements (compare with N). Arrow in L points to fusion between Meckel's cartilage (m) of arch 1 and the transformed ceratohyal (ch) of arch 2, an ultimate sign of segmental identity. Note the variable loss of cartilage dorsal of the foramen (f) (K,L), the reduction of the symplectic extension (sy) of the transformed hyosymplectic (hs) and its fusion with the interhyal (ih) (K), which is an arch 2-specific linker element absent in arch 1 (J), giving the hyosymplectic a spatial organization more similar to that of the palatoquadrate (pq). (M,N) Lateral views of head region ventral to eyes after in situ hybridization for bapx1, a first arch joint marker. Arrow points to ectopic bapx1 expression in arch 2 of the brpf1 mutant. (O,P) Lateral views of head region posterior to eyes after immunostaining of pharyngeal muscles with anti-MF20 antibody. (Q-T) Lateral (Q,R) and ventral (S,T) views of heads after staining of bone matrix with Alizarin Red (AR). Arrows point to absent ossification in ceratohyal (ch, ventral element; R) and hyomandibula (hm, dorsal element; T) of arch 2 in the mutant. In addition, the branchiostegal rays (bsr) and the opercle (op) dermal bones associated with the ventral and dorsal element of arch 2, respectively, are absent (arrowhead in R) or reduced. Furthermore, ceratobranchials (cb) of arches 3-6 display ectopic central ossifications (T), as in the wild-type ceratohyal of arch 2 (S). By contrast, arch 7 appears normal (S,T), with characteristic pharyngeal teeth formation (Van der Heyden et al., 2001). (U) Genetic and physical map of the t20002 allele of zebrafish brpf1. The three brpf1 exons on genomic fragment NA5599 are indicated in red. (V) Schematic of predicted wild-type and t20002, b943 and t25114 mutant Brpf1 proteins, with the C2H2, PHD finger, bromo and PWWP domains in different colors. am, adductor mandibulae; bb, basibranchial; ih, interhyal; lap, levator arcus palatini. |
|
Brpf1 regulates segmental identity by maintaining anterior Hox gene expression. Whole-mount in situ hybridizations with the probes indicated bottom left at the stages indicated bottom right; genotypes and treatment of zebrafish embryos as indicated in upper right corners. (A-L) Lateral views; (M-P) ventral views. (A-D) Hox gene expression in wild type (WT). Hox-expressing hindbrain rhombomeres (r) and arch-forming cranial neural crest (CNC) (2-7) are indicated. sc, spinal cord. (E-H) Absent or reduced Hox gene expression in brpf1 mutants (-/-). Arrow in H indicates the remaining hoxb3a expression in the posterior CNC. (I-L) Partially rescued Hox gene expression in the hindbrain (I, arrow) and the CNC (J-L, arrows) of brpf1 mutants injected with mouse Brpf1 mRNA. (M-P) The bimandibular phenotype of the brpf1 mutant (N) can be overcome by injection of hoxb1a mRNA. (O) hoxb1a-injected wild-type embryo lacking bapx1 expression, indicative for bihyoid phenotype [compare with Hunter and Prince (Hunter and Prince, 2002)]. (P) hoxb1a-injected brpf1 mutant with bihyoid pattern on left side and wild-type pattern on right side. Arch numbers are indicated. |
|
Expression pattern and cell-autonomous function of brpf1 in zebrafish CNC. Staining with reagents indicated at lower right at the stages indicated upper right. Numbers of pharyngeal arches are indicated (1-7). (A-J) Wild-type embryos; (K) brpf1b943 mutant (-/-); (L-O) mutant transplanted with wild-type cells (WT → -/-). (A) Dorsal view; (B-E,J-O) lateral views; (F-H) horizontal section; (I) longitudinal section. (A-D) At 26 hpf, brpf1 is co-expressed with the CNC marker dlx2a (A) and with fli1a (D), stained by anti-GFP immunostaining of tg(fli1a:EGFP) embryo (Isogai et al., 2003). brpf1-positive cells between CNC include pharyngeal endoderm [D; compare with Fig. 1A in Crump et al. (Crump et al., 2004)]. (E-I) At 55 hpf, sox9a-positive chondrocytes of cartilage condensates (cc; H) (Yan et al., 2002) and pax9a-positive pharyngeal endodermal cells (pe; G) (Nornes et al., 1996; Okabe and Graham, 2004) lack brpf1 expression, which, however, is strongly expressed in p63 (tp63 - ZFIN)-positive cells (Carney et al., 2007) of the pharyngeal ectoderm (pec; F-H), in the oral ectoderm (oe; I) and in facial ectoderm ventral to arches 1 and 2 (vfe; I) (Crump et al., 2006). (J-O) Analysis of chimeric embryos with rhodamine-dextran (RD)-labeled (N) and tg(fli1a:EGFP)-positive (M) wild-type cells integrated in the CNC of a brpf1 mutant host [for procedure, see Crump et al. (Crump et al., 2006)]. Only wild-type, not adjacent mutant CNC, cells display hoxa2b expression (arrows in L and M; n=3/3). g, gut; op, opercle. |
|
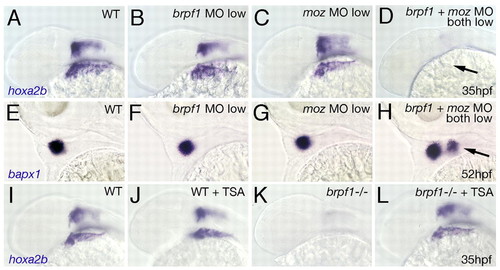
Genetic interaction of Brpf1 and Moz and rescue of the brpf1 mutant phenotype by TSA treatment. (A-H) Synergistic enhancement of phenotypes caused by partial loss of Brpf1 and Moz. hoxa2b (A-D; 35 hpf) and bapx1 (E-H; 52 hpf) in situ hybridizations of zebrafish larvae after single or double injections of low amounts of MOs, as indicated in upper right corners. Lateral views. Arrow in D points to absent hoxa2b expression in CNC. Arrow in H indicates ectopic bapx1 expression domain in arch 2. (I-L) Rescued hoxa2b expression in the brpf1 mutant (-/-) after TSA treatment (compare L with K), whereas expression in treated wild-type siblings remains largely unaltered (compare J with I). |
|
Co-localization and physical interaction of Brpf1 and Moz. (A-D) Brpf1 co-localizes with Moz. Immunofluorescent staining of interphase HEK 293 cells after co-transfection with the indicated versions of GFP-Brpf1 (left panels; green) and FLAG-Moz (middle panels; red), counterstained with DAPI (for DNA; blue); merged images are shown in right-hand panels. Full-length Brpf1 co-localizes with wild-type Moz (A) and with HAT-negative Moz-G675E (B) in a punctate pattern on interphase nuclei. Co-localization is abolished when Brpf1 is N-terminally truncated (C). N-terminal fragment of Brpf1 co-localizes with Moz, but displays a more diffuse distribution (D). (E,F) Schematic structures and co-localization/immunoprecipitation properties of full-length Brpf1, full-length Moz (E), and the various truncations used (F). G-I) Brpf1 physically associates with Moz. (G) Co-IP of full-length Brpf1 and wild-type or HAT-negative Moz(G657E) from co-transfected cells with anti-FLAG (Moz) antibody (left) or anti-HA (Brpf1) antibody (right), analyzed in western blots (upper panels) with the specified antibodies, or assayed for HAT activity on core histones (lower panels). (H) Co-IP of full-length FLAG-Moz and various GFP-Brpf1 deletion constructs with anti-FLAG or anti-GFP antibodies, followed by analysis of complex formation (upper panel) and control for Brpf1 expression levels (lower panel) via anti-GFP western blotting. (I) Co-IP of full-length Moz or C-terminally truncated MozN and various HA-tagged versions of the N-terminal fragments of Brpf1 using anti-FLAG antibody, analyzed in anti-HA western blots. Lower panel shows input control. Brpf1 aa 1-245 fragments that have histidine or cysteine mutations in the zinc-finger domain can still co-precipitate with Moz (lanes 5, 6), whereas the aa 1-149 fragment with an intact zinc finger cannot (lane 4). Scale bars: 5 μm in B; 2.5 μm in D. |
|
The PWWP domain is required for association of Brpf1 with metaphase chromosomes. (A-G) Immunofluorescent staining of mitotic HEK 293 cells transfected with the indicated GFP-Brpf1 constructs (A-E; green) and FLAG-Moz (F,G; red). (A,F) Spreads of metaphase chromosomes. Right panels of A-E and F,G show merged images with DAPI staining of DNA (blue). Full-length Brpf1 displays punctate distribution along metaphase chromosomes (A), whereas in intact nuclei, localization is concentrated in fewer, but still distinct domains of the DNA (B). Truncated Brpf1 lacking the PWWP domain (C) and a Brpf1 fragment containing the PHD domain and the bromodomain (D) are excluded from mitotic chromosomes, whereas a Brpf1 fragment containing the bromodomain and the PWWP domain co-localizes with DNA (E) in a similar manner to full-length Brpf1 (B). (F,G) In contrast to Brpf1 (A,B), no chromatin association is apparent for Moz in metaphase chromosome spreads (F) and in intact mitotic nuclei (G). (H) Schematic structures and chromosome-targeting properties of the full-length and truncated versions of Brpf1. (I-L) Immunofluorescent staining of mitotic HEK 293 cells, revealing that full-length Brpf1 (I-K; green) and the fragment containing the bromodomain and PWWP domain (L; green) co-localize with the active chromatin markers H2AK5Ac (I,L; red) and H3K4me1 (J; red), but not with the inactive chromatin marker H3K9me3 (K; red). Left panels are counterstained with DAPI (blue); merged images are shown in right-hand panels; regions with strong co-localization (yellow) are indicated by arrows. Scale bars: 2.5 μm in A; 5 μm in B,I-L. |
|
The bromodomain and PWWP domain bind histones. (A) Loading controls of GST-fused recombinant domains of Brpf1 (PHD finger, bromodomain, or PWWP domain) used in histone-binding assays (B-D). Relevant bands are indicated with asterisks. (B) Coomassie-staining of histones retained on glutathion beads without (A) or with (B) indicated GST-Brpf1 domains. Left lane shows 10% input of core histones used per assay. (C) Binding of purified H2A or H2B from calf serum with indicated GST-Brpf1 domains, analyzed by Coomassie staining. (D) Binding of core histones from untreated or butyrate-treated HeLa cells with indicated GST-Brpf1 domains, analyzed by western blotting with anti-H2AK5Ac (upper panel) or anti-H2A (lower panel) antibodies. The PWWP domain binds regular and hyperacetylated H2A equally well (compare lanes 5 and 6 of lower panel), whereas the bromodomain preferentially binds hyperacetylated H2A (compare lanes 3 and 4 of lower panel). This is also reflected in the higher relative signal intensity obtained with the anti-H2AK5Ac and the anti-pan H2A antibodies (compare upper and lower bands of lane 4 with those of lanes 6 and 8). |
|
Analysis of bpfr1 mutations and antisense MO. (A) Sequencing profiles of brpf1 genomic fragment from wild-type (+/+), heterozygous (+/-) and t20002 mutant (-/-) animals. In the mutant allele, TAC is mutated to a TAA stop codon, as indicated by an asterisk. (B) Sequencing profiles of brpf1 genomic fragment from wild-type (+/+, upper panel) and t25114 mutant animals (-/-, lower panel). The t25114 mutation generates a new splice acceptor site within the intron (AG), which is preferentially used. This leads to a 10 bp insertion (indicated in gray) and a frameshift in the cDNA. (C-E) The t25114 mutant brpf1 transcript and the resulting C-terminally truncated Brpf1 protein are stable. (C) Whole-mount in situ hybridization with brpf1 probe of wild-type (left) and t25114 homozygous embryo at 48 hpf. Embryos were genotyped after photography. (D) Semi-quantitative RT-PCR to amplify brpf1 (upper panels) or, as control, ef1α (lower panels) fragments from cDNA of single (left) or pooled (right) wild-type (WT) or t25114 homozygous larvae (-/-) at 120 hpf, when the phenotype was morphologically visible. Mutant bands were cloned, over 50 clones were sequenced and all found to contain the 10 bp insertion (data not shown). PCR primers used were: brpf1-F, TTCTTCACTGAGCCCGTACC and -R, GGGGACCAGAGACTTTAGGG; ef1α-F, TCACCCTGGGAGTGAAACAGC and -R, ACTTGCAGGCGATGTGAGCAG. (E) Anti-GPF western blot analysis of protein extracts from zebrafish embryos (10 hpf) after injection of plasmid DNA encoding GFP fusion proteins of full-length Brpf1 or C-terminally truncated Brpf1 lacking the PWWP domain (left). Full-length and truncated proteins are present in comparable amounts. (Right panel) Ponceau Red staining of nitrocellulose filter as loading control. (F-I) The three mutant brpf1 alleles display skeletal defects of similar strengths. Panels show Alcian Blue staining of heads at 120 hpf, ventral views. Numbers of pharyngeal arches and basihyal (bh) of second arch are indicated in F. Note that the t25114 allele (H), which only lacks the C-terminal PWWP domain, completely lacks the basihyal and the hypobranchials of arches 3 and 4, similar to the potential null allele t20002 (G; compare with Fig. 1G). (J-L) The brpf1 splice donor MO efficiently blocks splicing of intron 1 in vivo. (J) Diagram demonstrating of the structure of unspliced brpf1 hnRNA and the positions of the MO and the primers used in K. Primer sequences are available upon request. (K) RT-PCR on cDNA from uninjected and brpf1 morphant larvae at 48 hpf, using the primer pairs indicated in J. The wild-type cDNA (first lanes) only gave a band of appropriate size with a primer pair from exons 1 and 2 (panel 1), but no band with a primer pair from exon 1 and intron 1 (panel 2), or with two intron-internal primers (panel 3), whereas the morphant cDNA (second lanes) yielded the opposite pattern, indicating that intron 1 was not spliced out. Lanes 3 contain -RT controls, ruling out contamination with genomic DNA. Row 4 shows ef1α loading controls. (L) Northern blot analysis with brpf1 probe of total RNA from wild-type (lane 1) or morphant (lane 2) embryos. Only the wild-type RNA shows a brpf1 band of the appropriate size (5 kb). No band was detected in the morphant RNA, indicating that most of the unspliced brpf1 transcripts are degraded. (Lower panel) Methylene blue staining of blot showing rRNA as loading control. |
|
hox expression is initiated normally in brpf1 mutants. (A,B) hoxb1a (lateral views), (C,D) hoxb2a (lateral views) and (E,F) hoxa2b (dorsal views) whole-mount in situ hybridizations of wild-type sibling (A,C,E) and brpf1 mutant (B,D,F) embryos at segmentation stages (15 hpf is equivalent to the 12-somite stage; 18 hpf is equivalent to the 18-somite stage). Anterior Hox genes are initially expressed in hindbrain and cranial neural crest of mutants. Note that hoxa2b transcript levels in neural crest streams are already reduced, whereas in the hindbrain they are still normal (E,F). Rhombomere numbers (r) and cranial neural crest streams (2=hyoid; 3-7=gill arches) are indicated. EXPRESSION / LABELING:
|
|
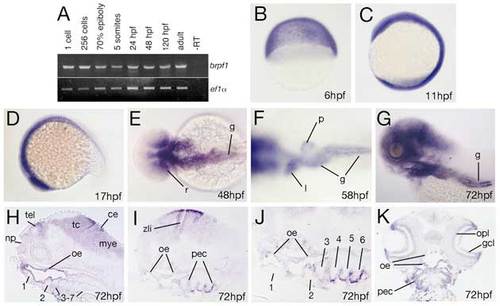
brpf1 displays early ubiquitous and later restricted expression in neuroectodermal, ectodermal and endodermal derivatives. (A) RT-PCR at indicated developmental stages for brpf1 and, as control, ef1α transcripts (for details, see legend to Fig. S1D). brpf1 is expressed at all stages investigated, from the 1-cell stage throughout adulthood (1-cell=0 hpf; 256-cell=early blastula stage=3 hpf; 70% epiboly=mid-gastrula stage=8 hpf; 5-somites=early segmentation stage=12 hpf). Transcripts detected during the first 4 hours of development, before the onset of zygotic transcription (Kane and Kimmel, 1993), are most likely maternally provided. To knockdown both maternal and zygotic brpf1 transcripts, we injected an antisense MO targeting the translational start site (in contrast to the described splice MO, which only targets zygotic transcripts). Preliminary results indicate that defects in segmental identity in such maternal-zygotic morphants are no more severe than in brpf1 mutants, with a regular initiation of anterior Hox gene expression (K.L. and M.H., unpublished; compare with Fig. S2). (B-K) brpf1 whole-mount in situ hybridization at stages indicated in lower right corners (6 hpf=shield stage=early gastrula; 11 hpf=3-somite stage; 17 hpf=16-somite stage). (B-D,G) Lateral views; (E,F) dorsal views; (H-J) longitudinal sections, (H) medial, (I,J) lateral; (K) horizontal section. Numbers of arches in H-J are indicated. ce, cerebellum; gcl, ganglion cell layer (retina); g, gut; l, liver; mye, myencephalon; np, nasal pit; oe, oral ectoderm; opl, outer plexiform layer (retina); pec, pharyngeal ectoderm; p, pancreas; r, posterior retina; tel, telencephalon; zli, zona limitans intrathalamica (Scholpp et al., 2006). |
|
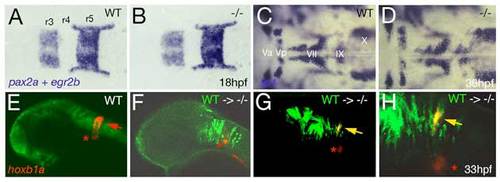
Loss of Brpf1 function in the hindbrain has a cell-autonomous effect on hoxb1a expression, but no obvious effects on anterior-posterior patterning. (A-D) Hindbrain patterning of brpf1 mutants appears normal. (A,B) egr2b (krox20) (Oxtoby and Jowett, 1993) and pax2a (Krauss et al., 1991) expression at 18 hpf, dorsal view of hindbrain region. In the brpf1 mutant (B; genotyped after photography), the width of rhombomeres r3, r4 and r5 is similar to that in wild-type siblings (A) (n=13/13). There was some variability (see r3), which, however, did not correlate with the brpf1 genotype. This is in contrast to the shifts obtained upon MO-based knockdown of hoxb1 genes, with a broadening of r3, whereas r4 and r5 are narrower than in uninjected siblings (McClintock et al., 2002). (C,D) In situ hybridization for isl1 transcripts (Inoue et al., 1994), 36 hpf, dorsal view of hindbrain region. Branchiomotor neurons of the trigeminal (V), facial (VII), glossopharyngeal (IX) and vagal nerves (X) are indicated. During normal development, cell bodies of the Vth nerve differentiate in r2 (Va) and r3 (Vp), where they remain during further development. Cell bodies of the VIIth nerve differentiate in rhombomers r4 and r5, followed by posterior migration to end up in r6 and r7 at 36 hpf (McClintock et al., 2002). brpf1 mutant (D; genotyped after photography) (n=21/12) and wild-type sibling show no significant difference in the patterning of isl1-positive branchiomotor cell bodies. The same results were obtained for mutants at 24 hpf, 28 hpf, 32 hpf and 40 hpf. This is in contrast to the phenotype observed in hoxb1 morphants, in which cell bodies of the Vth nerve remain in lateral positions of r4/5 (McClintock et al., 2002). Also in moz mutants, no defects during early rhomobomere patterning could be detected, while branchiomotor defects were much weaker and less penetrant than in hoxb1 morphants (Miller et al., 2004). Consistently, we found that Hox gene expression in the hindbrain of brpf1 mutants was much less affected than in the cranial neural crest (CNC) (see Fig. 2E-H and Fig. S2E,F). Together, this suggests that in the hindbrain, Brpf1/Moz activity and/or maintenance of anterior Hox gene expression is less critical for segmental identity determination than in the CNC. (E-H) Brpf1 promotes hoxb1a expression in hindbrain r4 cells in a cell-autonomous fashion. Fluorescein-dextran (Molecular Probes) was injected into wild-type donor embryos at the 1- to 4-cell stage. For hindbrain transplants, labeled cells were taken from the respective region (Kimmel et al., 1990) of shield stage (6 hpf) donor embryos and homotopically transferred into unlabeled shield stage brpf1 morphant hosts. Chimeric embryos were fixed at 35 hpf and in situ hybridized for hoxb1a, using Fast Red (Roche) as substrate. Subsequently, transplanted cells were stained by anti-fluorescein immunostaining. Chimeric embryos were analyzed by confocal laser-scanning microscopy (Zeiss LSM510 META). Lateral views of heads of wild-type (E) or brpf1 morphant embryos with transplanted wild-type cells (green; F-H). (H) A higher magnification of G. hoxb1a expression (in red) in ventral medial nucleus, which serves as internal control, is indicated by red asterisks. Chimeric embryos with wild-type cells anterior and posterior to r4 lacked hoxb1a expression (F; n=12/12), whereas wild-type cells within r4 were strongly hoxb1a-positive (H; yellow arrows; n=4/4). Note that hoxb1a expression remains absent in brpf1 morphant cells adjacent to hoxb1a-positive wild-type cells, ruling out the possibility that Brpf1 acts via a Hox expression-activating extracellular signal such as retinoic acid, which has a posteriorizing effect on Hox gene expression and segmental identity (Glover et al., 2006). EXPRESSION / LABELING:
|
|
Brpf1 in pharyngeal endoderm is neither necessary nor sufficient for segmental identities of skeletal elements of pharyngeal arches. Donor embryos were injected with Fluorescein-dextran (FD; Molecular Probes) and taramA* mRNA, encoding a constitutively active Nodal type I receptor that drives cells into an endodermal fate (David and Rosa, 2001). Upon transplantation into animal regions of host embryos, these cells directly ingress and form pharyngeal endoderm (David and Rosa, 2001), largely replacing endogenous anterior endodermal cells. (A,B) Fluorescent in situ hybridization for bapx1 transcripts (Fast Red), staining joint cells of arch 1 character, and anti-fluorescein immunostaining to visualize transplanted cells in the pharyngeal endoderm (green); 52 hpf; lateral views, confocal sections (Zeiss LSM510 META). brpf1 mutants with wild-type pharyngeal endoderm at the level of arch 2 display ectopic bapx1 expression like non-chimeric mutants (compare A with Fig. 1N; n=3/3), whereas wild-type embryos with brpf1 morphant pharyngeal endoderm lack bapx1 expression in arch 2, like non-chimeric wild-type animals (n=7/7; compare B with Fig. 1M). (C-F) Alcian Blue staining of head skeleton at 120 hpf. (C,D) Ventral views; (E,F) lateral views. Animals had been selected at 26 hpf, based on the abundance of fluorescein-positive cells in the pharyngeal endodermal cells (>50% of cells positive; not shown). Chimeric brpf1 mutants with wild-type pharyngeal endoderm show skeletal defects like non-chimeric mutants (n=4/4), including absence of basihyal (bh) (arrowhead in C; compare with Fig. 1D) and fusion of Meckel’s cartilage (m) and ceratohyal (ch) (arrowhead in E; compare with Fig. 1L). Conversely, chimeric wild types with brpf1 morphant pharyngeal endoderm are indistinguishable from non-chimeric wild-type animals (compare D with Fig. S1F, and F with Fig. 1J; n=12/12). hs, hyosymplectic; pq, palatoquadrate. |
|
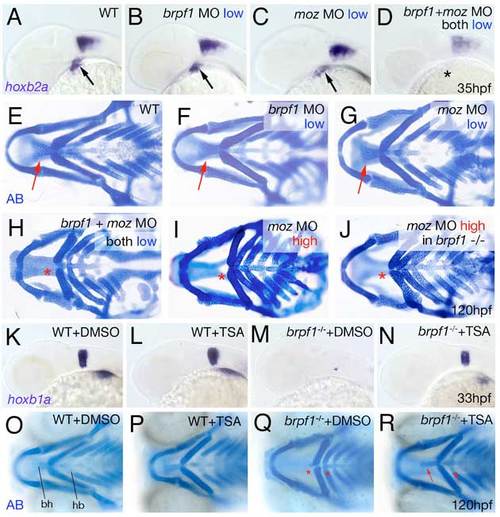
Brpf1 genetically interacts with Moz and brpf1 mutant defects are alleviated by HDAC inactivation with TSA. (A-D) hoxb2a in situ hybridizations at 35 hpf, lateral views. Arrows point to hoxb2a expression in cranial neural crest of wild-type embryo (A) and embryos injected with low amounts of brpf1 MO (B) or moz MO (C); asterisk indicates absent hoxb2a expression in embryo co-injected with the same low amounts of both MOs (D). (E-J) Alcian Blue staining of head skeletons at 120 hpf, ventral view. Arrows point to basihyal in wild-type (E), and larvae injected with low amounts of brpf1 MO (F) or moz MO (G). Asterisks indicate absent basihyal in larva co-injected with low amounts of brpf1 and moz MO (H), in strong moz morphant (I) and in brpf1 mutant injected with high amounts of moz MO (J). Co-injection of low doses of brpf1 MO and moz MO, which in single injections did not cause any phenotypes (compare F and G with E), caused defects as severe as in strongest brpf1 or moz morphants (compare H with I; n=11/13). By contrast, brpf1 mutants injected with highest amounts moz MOs showed a phenotype no more severe than that of moz single morphants (compare J with I; n=20/20). (K-N) TSA treatment enhances hoxb1a expression in r4 of both wild-type and brpf1 mutant embryos. hoxb1a in situ hybridizations at 33 hpf, lateral view of head region. TSA-treated wild-type embryo (L) displays slightly stronger hoxb1a expression than DMSO-treated sibling (K; n=27/35). TSA-treated brpf1 mutant displays recovered hoxb1a expression in r4 (N; embryo genotyped after photography; n=11/11), whereas expression remains absent in control mutant treated with DMSO (M). (O-R) TSA treatment from 20-33 hpf significantly ameliorates the later cartilage defects of brpf1 mutants. Alcian Blue staining of head skeletons at 120 hpf, ventral views. In O, basihyal of arch 2 (bh) and hypobranchials (hb) of arch 3 are indicated. The head skeleton of the TSA-treated wild-type larva (P) looks similar to that of the DMSO-treated control sibling (O). The TSA-treated brpf1 mutant (R) displays a significant amelioration of skeletal defects, including formation of hypobranchials and a normally sized basihyal (red arrows; animal genotyped after photography; n=26/33). By contrast, both elements remain absent or strongly reduced in control mutant treated with DMSO (Q; red asterisks; n=9/9). Stronger or longer TSA treatments interfered with additional developmental processes requiring HDAC activity and caused defects comparable to those of zebrafish hdac1 mutants (Pillai et al., 2004), including more severe craniofacial abnormalities, which masked the brpf1-specific traits. |
|
Brpf1-GFP displays PWWP domain-dependent chromatin association in zebrafish embryos. Zebrafish embryos were injected at the 1-cell stage with mouse full-length GFP-Brpf1 or GFP-Brpf1ΔPWWP plasmids, as also used for Fig. S1E, Figs 5 and 6, and fixed at mid-gastrula stages (7-9 hpf). GFP was visualized by immunofluorescence, as described for transfected HEK 293 cells (Figs 5 and 6), DNA was counterstained with DAPI, and embryos were analyzed by confocal microscopy (Zeiss LSM510 META). (A-C) Full-length Brpf1; (D) Brpf1ΔPWWP. (A) Interphase, (B) early mitosis, (C,D) late mitosis, with the two sets of sister chromosomes clearly separated. Full-length Brpf1 is localized at discrete sites of the chromatin of zebrafish cells both in interphase (A) and mitosis (B,C); compare with Fig. 5A and Fig. 6B for HEK 293 cells. (C,D) In contrast to full-length Brpf1 (C), Brpf1ΔPWWP lacking the PWWP domain is largely excluded from the chromatin (D); compare with Fig. 6D. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|