- Title
-
Redundancy and evolution of GATA factor requirements in development of the myocardium
- Authors
- Peterkin, T., Gibson, A., and Patient, R.
- Source
- Full text @ Dev. Biol.
|
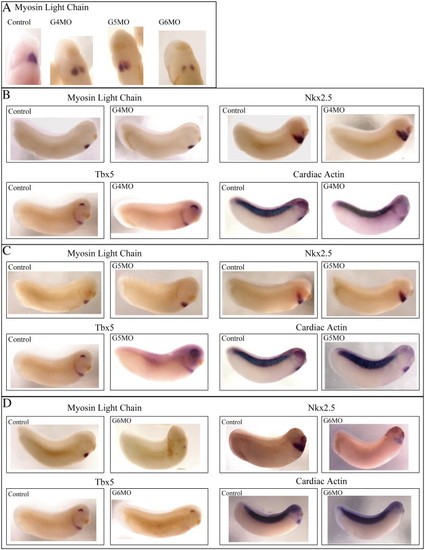
Cardia bifida is evident in GATA4, 5 and 6 morphants, but cardiac gene expression is only affected in GATA6 depleted Xenopus embryos. (A) Cardiac tissue stained for MLC fails to migrate to the midline in embryos injected singly with GATA4, 5 or 6 morpholinos. (B, C) Expression levels of MLC, Nkx2.5, Tbx5 and CA remain unchanged in GATA4 and 5 morphants compared with control uninjected embryos at stage 28. (D) Expression levels of MLC, Nkx2.5, Tbx5 and CA are substantially decreased in GATA6 morphants at stage 28. |
|
Functional redundancy between GATA4, 5 and 6. (A) Xenopus embryos were injected singly and in combination with GATA4, 5 and/or 6 morpholinos, harvested at stage 28 and analysed by whole mount in situ hybridisation for MLC and Nkx2.5 expression. Morphant embryos were classified into four classes: wild type (type +, light blue bar), mild down regulation of MLC or Nkx2.5 (type -, mid blue bar), strong down regulation (type --, purple bar) and no expression (type ---, dark blue bar). (B, C) Graphical representations of the proportion of embryos in each class. |

Induction of cardiac precursor gene expression is unaffected in morphant Xenopus neurulae. (A) Nkx2.5 was expressed normally at stage 16/17 when GATA4, 5 or 6 were depleted individually or in combination. (B) Cryostat sections confirmed that expression of Nkx2.5 in the cardiac mesoderm (delineated by red dashed lines) was not affected. (C) Expression of Nkx2.3, which unlike Nkx2.5 is restricted to the cardiac mesoderm (D) also remained unchanged in the triple morphants, as does eHAND (E). (Red dashed lines mark the cardiac precursors, remaining stain reflects expression in the blood island mesoderm). |
|
GATA5 and 6 are essential but GATA4 is redundant in zebrafish myocardium. Zebrafish embryos were injected with GATA4, 5 and/or 6 morpholinos individually or in combination and analysed by whole mount in situ hybridisation at 26 hpf for the expression of Nkx2.5 (A, B), vmhc (C, D) and cmlc2 (E, F). Anterior views: red brackets and arrowheads identify the cardiac expression. Embryos were classified into unaffected (type +), downregulated (type -) and absent (type --) for expression of nkx2.5 (B), vmhc (D) and cmlc2 (F) and graphically represented. The effect on all three cardiac genes studied was similar for each injection. Depletion of GATA4 alone had little impact whereas loss of GATA5 or 6 caused a reduction in the expression of all three. Combinatorial ablation of GATA4 + 5 and GATA4 + 6 caused a further reduction in expression compared to the ablations of GATA5 or GATA6 alone thus demonstrating functional redundancy for GATA4 in the expression of these cardiac genes. GATA5 + 6 ablation resulted in complete loss of expression demonstrating an additive effect for these two GATAs. |
|
Expression of nkx2.5 and GATA4 in zebrafish morphants of GATA4, 5 and/or 6 separately and in combination. Flat mounts, dorsal views (A) A, anterior; P, posterior; D, dorsal; V, ventral. (B) As seen at 26 hpf (Fig. 4), GATA4 morphants at 10 somites showed normal expression of nkx2.5, whereas GATA6, and more severely GATA5, morphants showed a reduction in expression. In combination morpholino injections, down regulation was exacerbated in each case, as seen at 26 hpf (Fig. 4), with expression being completely absent in embryos depleted for both GATA5 + 6. (C) Loss of GATA5 reduces the expression of GATA4 and there is a complete loss of GATA4 expression when GATA5 and 6 are depleted in combination (G5 + G6MO). The black brackets indicate the cardiac expression. Krox20 and MyoD are stained in red and were used as landmarks. Krox20 is expressed in rhombomeres 3 and 5, MyoD was used to confirm the number of somites in the embryo. EXPRESSION / LABELING:
PHENOTYPE:
|
|
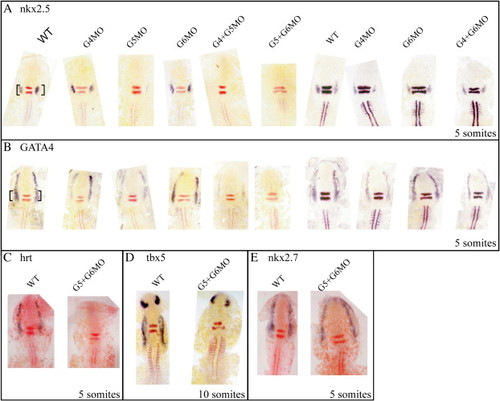
Cardiac induction requires GATA activity in zebrafish. Flat mounts: dorsal views. (A) nkx2.5 expression at 5 somites requires GATA5 and redundantly GATA4 and GATA6. The loss of GATA4 and/or GATA6 alone has little effect on the initiation of nkx2.5. (B) GATA4 expression at 5 somites requires GATA5 and redundantly GATA6. GATA4 is also required to maintain its own expression (B). Hrt/tbx20 expression is lost in the GATA5 and 6 double morphants (C) as is Tbx5 expression at 10 somites (D). (E) nkx2.7 expression remains unchanged in embryos injected with GATA5 and 6 morpholino. Note that GATA5 and 6 double morphants knock out GATA4 expression and therefore represent a triple knockdown. The black bracket indicates the cardiac expression. EXPRESSION / LABELING:
|
|
GATA4 MO specifically inhibits the translation of GATA4 RNA and GATA5 MO inhibits the production of endogenous GATA5 RNA. (A) GATA4 MO sequence and the ATG region to which it binds. (B) A Western blot to show the inhibition by GATA4 MO of translation of exogenous HA-tagged GATA4 RNA injected into Xenopus animal caps. (C) A Western blot to show that GATA4 MO specifically inhibits translation of GATA4 RNA injected into Xenopus oocytes, and has no effect on GATA5 or GATA6 translation. (D) Genomic structure of the GATA5 gene. Exons (ex) depicted as boxes, intron sizes not to scale. Splice MO binding site (red line) and primer binding sites for RT–PCR analysis (black lines) are shown. (E) The amplified 260 bp GATA5 fragment is present at a much lower level in the RNA extracted from embryos injected with 25 ng MO, indicating that there is a reduced level of correctly spliced GATA5 in the injected embryos. (F) Analysis of vmhc expression at 26 hpf (dorsal anterior view) and nkx2.5 expression at 10 somites (flat mount, dorsal view) shows that 25 ng GATA5 MO effectively phenocopues the fausttm236a mutant phenotype, black brackets identify cardiac expression. |
Reprinted from Developmental Biology, 311(2), Peterkin, T., Gibson, A., and Patient, R., Redundancy and evolution of GATA factor requirements in development of the myocardium, 623-635, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.