- Title
-
SCL-GFP transgenic zebrafish: In vivo imaging of blood and endothelial development and identification of the initial site of definitive hematopoiesis
- Authors
- Zhang, X.Y., and Rodaway, A.R.
- Source
- Full text @ Dev. Biol.
|
Expression of GFP in the scl-PAC-GFP line completely recapitulates that of endogenous scl (see also Supplementary Fig. 2, online) The figures are composites of DIC and fluorescence images. Except where stated otherwise, all figures are lateral views with anterior to the left. (A) 11 Somites (14.5 hpf), dorsal view of trunk, anterior to left. GFP is expressed in the blood and endothelial precursors in the posterior lateral plate mesoderm (PLM). (B) 14 Somites (16 hpf), dorsal view of head, anterior to left. GFP is expressed in primitive macrophages (arrow) migrating out onto the yolk cell, and the precursors of the head vasculature, including the endocardium (arrowhead). (C) 16 Somites (17 hpf), dorsal view of trunk, anterior to left. GFP expression converges, under the somites, towards the midline. (D) 18 Somites (18 hpf). The GFP-expressing cells in the trunk have formed the intermediate cell mass (ICM, arrow) ventral to the notochord. (E) 24 hpf. As well as the ICM, GFP is expressed in the developing vasculature including the mid cerebellar vessel (arrowhead) and the endocardium and aortic arches. (F) 24 hpf, higher magnification of the trunk. GFP is expressed in the differentiating erythroid cells in the ICM (large arrow) and the endothelium of the segmental vessels (small arrows) that sprout from the dorsal aorta. (G) 48 hpf. GFP is expressed in the CNS, with particularly strong expression in the midbrain (arrowhead) and part of the diencephalon, and in interneurons in the ventral hindbrain and spinal cord. Expression is also visible in the ICM after the primitive erythroid cells have entered circulation (arrow). The fluorescence in the region of the yolk cell results from the flow of blood returning to the heart over the anterior surface of the yolk, along with autofluorescence of the yolk granules. (H) 11 dpf. GFP expression in the CNS and ICM is maintained at the latest stage we have examined in detail. Autofluorescence is also seen in the gut. (I) 11 dpf, high magnification view of the pronephric region. Round GFP-expressing cells are visible in this region, the site of hematopoiesis through adulthood. The diffuse fluorescence in the lower half of the micrograph is autofluorescence in the gut. |
|
Migration of blood and endothelial precursors. Projections of confocal images from timelapse experiments performed on scl-PAC-GFP transgenic embryos scatter labeled with nuclear-localized H2B-mRFP1 fusion. (A–D) Migration of blood and endothelial precursors from the PLM to the trunk midline. Dorsal view of trunk, anterior to top, dotted line indicates embryonic midline. 3-h Timelapse, 12-min intervals. (A) Projection of GFP and RFP expression at the start of the timelapse (11 somites, 14.5 hpf). The GFP cells are in the PLM. (B) Projection of GFP and RFP expression at the end of the 3-h timelapse (16 somites). GFP expression has begun to reach the midline in the anterior part of the trunk. (C) Colocalization of GFP and RFP expression identifies the nuclei of a subset of the GFP-expressing cells. (Image processed using the ‘RG2B colocalization’ plug-in for ImageJ). (D) The positions of these nuclei at the end of the timelapse are shown, along with the paths they took during their migration. Several of the cells have reached the midline. It is generally the case that the more rostral cells migrate first. However, the migration of the cells is not completely synchronous, and the paths of some nuclei cross. For example, the most rostral cell tracked (orange path) migrates later than cells immediately posterior to it, and crosses their paths. This implies that the cells are migrating independently rather than moving as a tissue en masse. (E–H) Migration of cells from the ALM domain of scl expression to form the head vasculature and yolk sac macrophages. Dorso-lateral view of head, anterior to the right. 460-min timelapse, 10-min intervals. (E) Projection of GFP and RFP expression at the start of the timelapse (13 somites, 15.5 hpf). The GFP-expressing domain is lateral to the midbrain and ventral to the mid- and hindbrain. (F) Projection of GFP and RFP expression at the end of the timelapse (approximately 20 somites). Cells were identified as macrophages or angioblasts by position, shape and arrangement relative to other cells. The ventral GFP-expressing cells are primitive macrophages that have migrated out onto the yolk sac, while the remainder are forming the head vasculature. (G) Colocalization of GFP and nuclear RFP at the start of the timelapse. Selected nuclei are indicated. Those that gave rise to angioblasts are labeled in shades of blue, and those that gave rise to macrophages are labeled in shades of red. (H) Positions of these nuclei at the end of the timelapse, together with the paths they followed. In this experiment, angioblasts were found in the positions of the lateral aorta (1:7, 1:9, 1:10), primordial hindbrain channel (1:17), primordial midbrain channel (1:13) and optic vein (1:1). The paths taken by five cells that gave rise to macrophages are also shown. In general, more posterior and medial cells gave rise to angioblasts while more anterior and lateral cells formed macrophages. However, occasional antero-lateral cells become angioblasts (e.g., 1:1) and some macrophages emerge from more medial positions (e.g., 2.4). |
|
The development of hematopoietic clusters during late embryogenesis. Panels A–J show paired lateral views (left panel DIC, right panel GFP fluorescence) in scl-PAC-GFP transgenic embryos. Panels A, C, E, G and I show mid-trunk, and B, D, F, H and J show the ventral tail. Panel K shows paired views of the mid-trunk of a 4 dpf fli1-promoter-GFP transgenic embryo. Panel L and M show DAF staining for globin expression in the trunk and tail, respectively, of a 4 dpf embryo. Red bars indicate the dorso-ventral positions and sizes of the lumens of the major artery (DA in trunk, CA in tail). Blue bars indicate the lumens of the major vein(s) (PCV in trunk, CV in tail). All images are centred on boundaries between somites in the adjacent paraxial mesoderm. (A) Soon after the primitive erythroid cells of the ICM have entered circulation (31 hpf), the floor of the DA and roof of the PCV are closely apposed, and consist of thickened endothelia. These endothelia express GFP (A′) as do the circulating erythroid cells (blurred fluorescence in the lumens of the vessels). (B) The ventral tail region contains the CA and the anastomosing vessels comprising the CV. GFP (B′) is expressed in these endothelia and in clusters of round cells interspersed among the venous network. (C) 2 dpf: the endothelia of the floor of the DA and roof of the PCV have thinned and occasional cells with a rounder morphology are visible between the endothelia. These express GFP (C′). (D) Multiple round cells expressing GFP (D′) are present between the CA and CV. (E) 3 dpf: the DA and PCV have separated. Occasional round GFP-expressing cells adhere to the ventral surface of the DA (small arrows), but the majority of such cells have started to form small clusters on the roof of the PCV (large arrows). These clusters are adjacent to the boundaries between somites. (F) GFP-expressing round cells occupy much of the tissue between the CA and PCV. (G) 4 dpf: the GFP-expressing clusters have expanded in size (typically 5–25 cells), and the ventral-most cells are in contact with the lumen of the PCV (see also panel I). (H) More of the GFP-expressing round cells are present ventrally (near the CV) than dorsally (near the CA). (I) 6 dpf: the trunk clusters have begun to reduce in size. Ventral cells project into the lumen of the PCV and are presumably entering circulation. Small cluster are seen as late as 11 dpf (data not shown). (J) The hematopoietic cells in the tail are all associated with the roof of the CV. This expression is also maintained through later embryonic development. (K) Expression of GFP under control of an endothelium-specific fli1 promoter labels cells surrounding the trunk clusters, but not the cells in the clusters themselves. This GFP-expressing endothelium appears continuous with that of the PCV. Note, this does not imply that the clusters do not express endogenous fli1, only that the endothelial-specific promoter contained in the fli1-GFP construct is not active in these cells. (L) 4 dpf, trunk: a subset of cells in the trunk clusters expresses globin, as visualized by DAF staining. Staining can also be seen in circulating erythrocytes, both in the DA (dorsally) and PCV (ventrally). (M) Similarly, some of the cells between the CA and CV also express globin. (N) Lateral view of a 3 dpf embryo (bright field). The size and positions of the micrographs labeled ‘trunk’ and ‘tail’ in this image and in Fig. 5 and Fig. 6 are indicated. (O) ‘Virtual transverse section’ through the trunk of a living scl-PAC-GFP transgenic embryo, reconstructed from a confocal stack. GFP fluorescence in green, autofluorescence (predominantly pigment cells on the embryo surface) in blue. The hematopoietic clusters are in the midline of the embryo, between the paired hypaxial somites. (P) Schematic to aid interpretation of the DIC and fluorescence images in this image and in Fig. 6 and Fig. 7. A DIC image of the trunk region of a 4 dpf embryo has been false-colored to indicate the most important features: hematopoietic cluster—green; dorsal aorta—red; PCV—purple; somite boundary—yellow. At this stage of development, the cluster lies adjacent to the somite boundary, and in contact with the roof of the PCV. There is an out-pocketing of the PCV at the site of the cluster. |
|
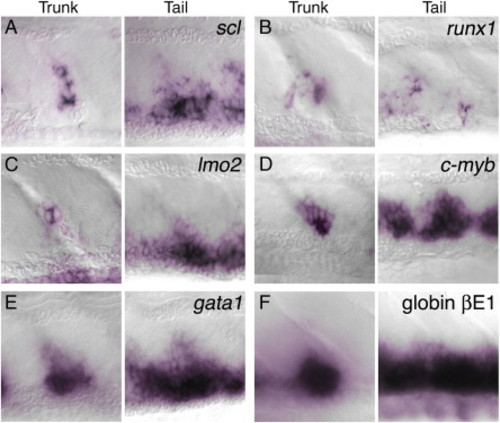
Expression of hematopoietic markers in the trunk clusters and ventral tail of 4 dpf embryos. Whole-mount in situ hybridization to show mRNA expression (purple) of scl (A); runx1 (B); lmo2 (C); c-myb (D); gata1 (E); and βE1 globin (F). This expression supports the identification of the trunk clusters as hematopoietic. Compared with the other markers runx1 expression is expressed in much fewer cells in the ventral tail. EXPRESSION / LABELING:
|
|
Treatments to block definitive hematopoiesis ablate the trunk hematopoietic clusters, but have less affect upon tail hematopoiesis. Panels show paired DIC and GFP fluorescence images of 4 dpf scl-PAC-GFP transgenic embryos. The lumens of the major vessels are indicated as in Fig. 4. Panels A, C, and E are lateral views in the mid-trunk, centred on a somite boundary. Panels B, D, and E show the ventral tail. Panels A and B show a control embryo from a TCDD experiment, treated with vehicle (0.1% DMSO) alone. Panels C and D are an embryo treated with 6 ng/ml TCDD at 12 hpf. Panels E and F are an embryo injected with 2 ng of a splice-blocking antisense morpholino oligonucleotide (MO) targeted to runx1. (A) A typical trunk hematopoietic cluster consisting of approximately 15 GFP-expressing round cells. Interestingly, this cluster is associated with one of the transient vessels that link the DA and PCV at this time (Isogai et al., 2001). This is the case for some, but not all, of the hematopoietic clusters at this stage. (B) The tail contains many hematopoietic cells, predominantly associated with the roof of the CV. (C) In embryos treated with TCDD, the formation of trunk hematopoietic clusters is ablated. In this embryo, only one GFP-expressing cell with round morphology was seen (arrow). All other GFP-expressing cells between the DA and PCV have disorganized endothelial morphology. (D) In the tail of TCDD-treated embryos there is a slight increase in GFP-expressing cells having endothelial morphology and a slight decrease in cells with hematopoietic morphology. However, plentiful hematopoietic cells are still detectable. (E) In runx1 MO-injected embryos, trunk hematopoietic clusters are completely ablated. Endothelial cell morphology was normal, but, interestingly, the separation of DA and PCV is reduced. (F) In the tail of runx1 MO-injected embryos, the number of GFP-expressing hematopoietic cells is marginally reduced. Endothelial morphology is apparently unaffected. PHENOTYPE:
|
|
The second GFP transgenic line, scl-6kUS-GFP lacks elements necessary for expression in definitive hematopoietic clusters. (A) 34 hpf scl-PAC-GFP embryo, and (B) 34 hpf scl-6kUS-GFP embryo, lateral views. In both embryos, GFP is expressed in the CNS and endothelium in identical patterns. In panel A, expression in the primitive erythrocytes is easily seen as they pass over the yolk cell and enter the heart (arrowhead). In panel B, there is much reduced expression in erythroid cells, but is still faintly visible (see also, panel F). (C and D) 4 dfp scl-PAC-GFP embryo, mid-trunk hematopoietic cluster. A typical cluster of approximately 15 cells is visible in the DIC image (C). Strong expression of GFP in these cells is clearly visible (overlay of fluorescent and DIC image) (E and F), 4 dfp scl-6kUS-GFP embryo, mid-trunk hematopoietic cluster. A cluster of approximately 13 cells is visible in the DIC image (E). No expression of GFP is visible in the cluster (overlay of fluorescent and DIC image). Faint expression in circulating primitive erythrocytes is visible in the lumen of the vessels. |
|
Colocalization of expression of endogenous scl message and GFP protein in scl-PAC-GFP transgenic embryos. scl message was detected by whole-mount in situ hybridization using the red fluorescent substrate, Fast Red (Sigma). Subsequently, GFP protein was detected using affinity-purified rabbit polyclonal antiserum to GFP (Torrey Pines Biolabs), followed by a goat anti-rabbit F(ab2)2 secondary antiserum labeled with AlexaFluor488 (Molecular Probes). Whole-mount in situ signal (red fluorescence) and immunofluorescence (green fluorescence) were imaged using a Nikon C1 confocal microscope. In general, expression of the GFP transgene coincides with the expression of the endogenous scl message. However, in tissues where scl expression has newly initiated, detection of the GFP protein is, as would be expected, delayed relative to detection of the scl message. (A) Lateral view of a 25-somite zebrafish embryo. Arrows indicate the regions imaged in panels B to G. (B–D) Intermediate cell mass, sagittal confocal slice. (B) Endogenous scl message. (C) GFP protein. (D) Overlay of panels B and C. GFP is co-expressed with the endogenous scl message in the mass of primitive erythroblasts as well as in the endothelium of the forming dorsal aorta (white arrow). (E–F) Ventral part of spinal cord, parasagittal confocal slice. (E) Endogenous scl message. (F) GFP protein. (G) Overlay of panels E and F. Expression of endogenous scl has recently initiated in interneurons in the ventral spinal cord. GFP protein can be detected in most (white arrows) but not all (white arrowhead) of these cells. (H–K) Dorsal view of head of 4 dpf embryo. Fluorescence images are Z-projections of confocal stacks. (H) Brightfield. (I) Endogenous scl message. (J) GFP protein. (K) Overlay of panels I and J. GFP protein is co-expressed with endogenous scl message in ventral hindbrain, optic tectum and diencephalon. |
Reprinted from Developmental Biology, 307(2), Zhang, X.Y., and Rodaway, A.R., SCL-GFP transgenic zebrafish: In vivo imaging of blood and endothelial development and identification of the initial site of definitive hematopoiesis, 179-194, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.