- Title
-
Visualization of two distinct classes of neurons by gad2 and zic1 promoter/enhancer elements in the dorsal hindbrain of developing zebrafish reveals neuronal connectivity related to the auditory and lateral line systems
- Authors
- Sassa, T., Aizawa, H., and Okamoto, H.
- Source
- Full text @ Dev. Dyn.
|
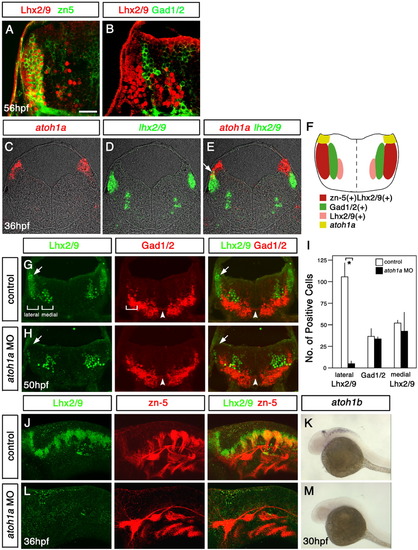
Two distinct classes of neurons in the dorsal hindbrain of zebrafish embryos. A,B: Immunostaining of coronal sections of the hindbrain at the level of the otic vesicle with the Lhx2/9 (A,B), zn-5 (A), and Gad1/2 (B) antibodies. Dorsal is to the top, lateral is to the left. The lateral-most neurons express both the zn-5 antigen and Lhx2/9, while a distinct neuronal population located medially to the zn-5(+)Lhx2/9(+) neurons express Gad1/2. C-E: Fluorescent in situ hybridization of serial coronal sections (10 μm in thickness) of the hindbrain at the level of the otic vesicle with the atoh1a (C,E) and lhx2/9 (D,E) probes. Dorsal is to the top. E is a composite figure of C and D. F: Schematic illustration of gene expression profiles in the dorsolateral hindbrain. Coronal view. G,H: Coronal sections of the hindbrain of the control (G) and atoh1a morpholino (MO) -injected (H) embryos at the level of the otic vesicle, immunostained with the Lhx2/9 and Gad1/2 antibodies. Dorsal is to the top. The arrows indicate the cluster of the lateral Lhx2/9(+) neurons, which is severely reduced in number in the atoh1a MO-injected embryos. The arrowheads indicate the Gad1/2(+) commissural fascicles. The brackets indicate the regions where the cell number was counted to collect data for I. I: Cell counts (mean + SD) for lateral Lhx2/9(+), Gad1/2(+), and medial Lhx2/9(+) neurons in control and atoh1a MO-injected embryos. Lhx2/9- and Gad1/2-immunopositive cells at the level of the otic vesicle were counted bilaterally on three independent sections (20 μm in thickness) derived from different embryos. Only the number of lateral Lhx2/9(+) neurons is significantly reduced in the atoh1a MO-injected embryos. Asterisk indicates P < 0.01, Student t-test). J-M: Expression of Lhx2/9, the zn-5 antigen and atoh1b in the control (J,K) and atoh1a MO-injected (L,M) embryos was examined by immunostaining (Lhx2/9 and zn-5 antibodies) and in situ hybridization (atoh1b antisense probe). Lateral view, dorsal is to the top, anterior is to the left. All three signals were specifically abolished in the dorsolateral hindbrain of the atoh1a MO-injected embryos. Scale bars = 20 μm in A,B, 40 μm in C-E, 50 μm in G,H,J,L, 250 μm in K,M. |
|
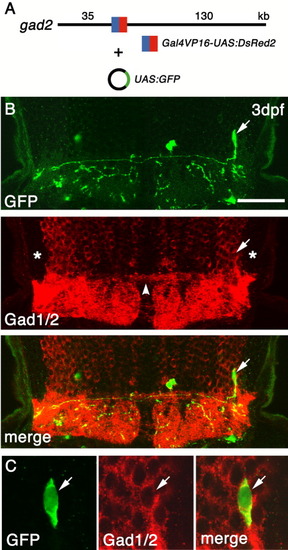
Visualization of a single Gad1/2(+) axon projecting to the contralateral hindbrain. A: Structure of the gad2 bacterial artificial chromosome (BAC) clone with Gal4VP16/UAS:DsRed2 inserted into the 5′-untranslater region (5′-UTR) of gad2. B: A coronal section of the hindbrain of an embryo injected with the modified gad2 BAC DNA and UAS:GFP plasmid DNA. Dorsal is to the top. A single axon of a green fluorescent protein-positive (GFP(+)) neuron (arrow) projects to the contralateral hindbrain. Asterisks indicate the regions likely occupied by the zn-5(+)Lhx2/9(+) neurons. Note the absence of Gad1/2 signal in this region. Note also the Gad1/2(+) commissural fascicles connecting the left and right lateral Gad1/2(+) neurons (arrowhead). C: The same single GFP(+) neuron as in B, at higher magnification, demonstrating that this neuron is also positive for Gad1/2. UAS, upstream activating sequences. Scale bars = 75 μm in B, 15 μm in C. |
|
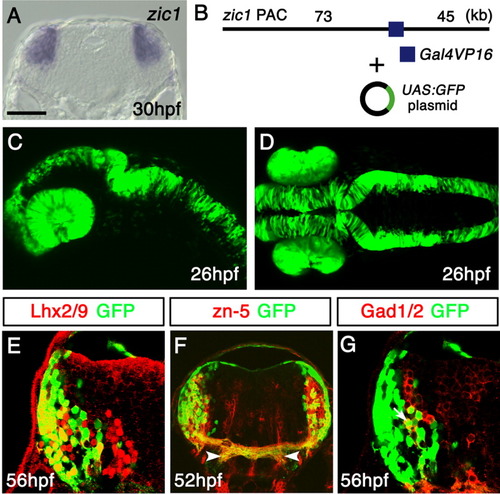
Green fluorescent protein (GFP) expression in the Tg(zic1:Gal4VP16/UAS:GFP) embryos. A: In situ hybridization of a 30-hours postfertilization (hpf) coronal section of the hindbrain at the level of the otic vesicle, using the zic1 probe. Dorsal is to the top. B: The structure of the DNA constructs used to generate the Tg(zic1:Gal4VP16/UAS:GFP) fish. The zic1 PAC with Gal4VP16 inserted into the 5′-untranslater region (5′-UTR) of zic1 was coinjected with the UAS:GFP plasmid. C,D: Lateral (C) and dorsal (D) views of the Tg(zic1:Gal4VP16/UAS:GFP) embryos at 26 hpf. GFP is expressed in the eyes and dorsal neural tube. E-G: Cross-sections of the hindbrain of the Tg(zic1:Gal4VP16/UAS:GFP) embryos stained with the Lhx2/9 (E), zn-5 (F), and Gad1/2 (G) antibodies. The lateral GFP(+) cells predominantly coexpressed Lhx2/9 (E) and the zn-5 antigen (F), while some of the medial GFP(+) cells coexpressed Gad1/2 (G). A representative double-positive cell is indicated with an arrow in G. (F) The axons of the GFP(+) neurons colocalized with the zn-5(+) commissural axons. These axons projected ventrolaterally after crossing the midline (arrowheads). They were connected with the longitudinal fascicles as shown in Figure 4B. UAS, upstream activating sequences. Scale bars = 60 μm in A, 100 μm in C,D, 25 μm in E,G, 50 μm in F. |
|
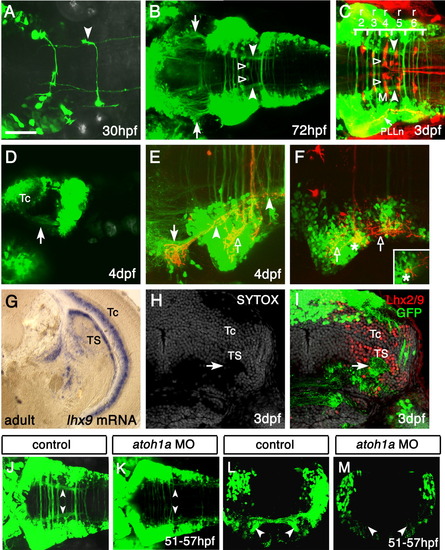
The zn-5(+)Lhx2/9(+) neurons project to the contralateral torus semicircularis (TS). A: Dorsal view of the hindbrain of a Tg(zic1:Gal4VP16/UAS:GFP) embryo at 30 hours postfertilization (hpf). The axon of a single green fluorescent protein-positive (GFP(+)) neuron can be seen turning rostrally after crossing the midline (arrowhead). Rostral is to the left. B: By 72 hpf, the GFP(+) axons formed a ladder-like structure, which consisted of many commissures and two longitudinal fascicles (filled arrowheads). The axon terminals of the two longitudinal fascicles appear to reach the lateral midbrain (arrows). Additionally, a pair of GFP(+) patches (open arrowheads) observed in rhombomere 4 (r4) around the initial axon segment of the Mauthner cell (M, compare with C) were probably Mauthner axon caps formed by the spiral fiber neurons and/or passive hyperpolarizing potential neurons (Lorent et al.,[2001]). The position of cell bodies providing the GFP(+) Mauthner axon caps was unclear. C: Reticulospinal neurons were visualized in the 3 days postfertilization (dpf) Tg(zic1:Gal4VP16/UAS:GFP) embryos by retrograde incorporation of tetramethylrhodamine-dextran. As in B, the longitudinal fascicles and Mauthner axon caps are indicated by the filled and open arrowheads, respectively. The majority of commissures were distributed from r2 to r6, and the caudal end of the longitudinal fascicle corresponded approximately to the caudal r6 border. Note the gross overlap of the GFP(+) neurons with the spreading of central nerves of the posterior lateral line (PLLn, arrow), which was also accidentally labeled by tetramethylrhodamine-dextran. M, Mauthner cell. D: Lateral view of the 4-dpf Tg(zic1:Gal4VP16/UAS:GFP) embryos. Anterior is to the left. The GFP(+) longitudinal fascicles appeared to terminate in the lateral midbrain region ventral to the optic tectum (arrow, compare with B). Tc, optic tectum. E,F: Dorsal views of a Tg(zic1:Gal4VP16/UAS:GFP) embryo with 1,1′, di-octadecyl-3,3,3′,3′,-tetramethylindo-carbocyanine perchlorate (DiI) applied on the contralateral dorsal hindbrain. Anterior is to the left. Images in E,F were obtained from the same embryo but at different dorsoventral focal planes. The image in E was obtained from the deeper (ventral) region, while the image in F was from the more superficial (dorsal) region. After crossing the midline, the DiI-labeled axons were segregated into the dorsal and ventral fascicles. E: The ventral fascicle turned rostrally, joined the anteriorly projecting longitudinal fascicle (filled arrowheads) and terminated in the GFP(+) region of the midbrain (arrow, compare with B). The posterior segment of the longitudinal fascicle (right filled arrowhead) derives from the anterior projection of caudal commissural axons, which are weakly labeled with the dye. F: The dorsal fascicle advanced straight, then bifurcated and terminated in the contralateral hindbrain with extensive branches (open arrows in E,F). Retrograde labeling of a cell body (asterisks) was also observed. A confocal section showed that the neuron was GFP(+) (inset). G: In situ hybridization of a coronal section of the adult midbrain, using the lhx9 probe. Dorsal is to the top, lateral is to the right. The expression of lhx9 is evident in the torus semicircularis (TS) and periventricular gray zone of the optic tectum (Tc). H,I: Immunostaining of a coronal section of the embryonic Tg(zic1:Gal4VP16/UAS:GFP) midbrain with the Lhx2/9 antibody. Cell nuclei (gray) are counterstained by SYTOX dye. The axon terminals of the GFP(+) longitudinal fascicles (arrow) were distributed through the entire mediolateral extent of the region expressing Lhx2/9. This staining pattern of Lhx2/9 in the embryo is essentially identical to that of lhx9 in adult fish (G), suggesting that the GFP(+) longitudinal fascicles formed by the zn-5(+)Lhx2/9(+) neurons project to the developing midbrain TS. J-M: Knockdown of Atoh1a impairs the formation of the lateral longitudinal fascicles (LLFs). J,K: Dorsal views of the control (J) and atoh1a MO-injected (K) Tg(zic1:Gal4VP16/UAS:GFP) embryos. In the atoh1a MO-injected embryos (K), the commissural fascicles are thin and LLFs (compare arrowheads in J,K) are absent. Anterior is to the left. L,M: Coronal sections of the hindbrain of the control (L) and atoh1a MO-injected (M) Tg(zic1:Gal4VP16/UAS:GFP) embryos. Dorsal is to the top. Commissural fascicles are very thin and LLFs are absent (compare arrowheads in L,M). UAS, upstream activating sequences. Scale bars = 50 μm in A, 100 μm in B, 75 μm in C, 100 μm in D, 50 μm in E,F,H,I, 200 μm in G, 80 μm in J,K, 60 m in L,M. EXPRESSION / LABELING:
|
|
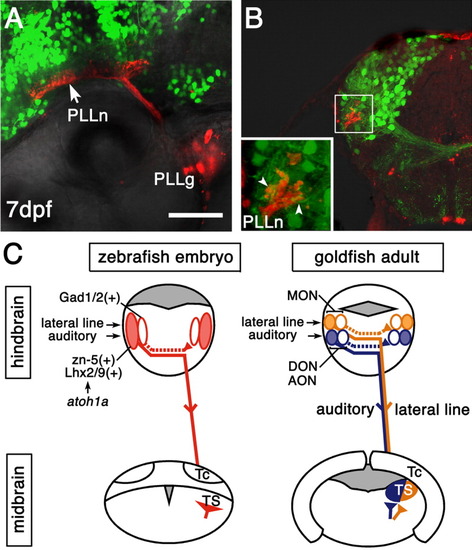
The zn-5(+)Lhx2/9(+) and Gad1/2(+) neurons might receive auditory and lateral line information. A,B: Posterior lateral line nerves (PLLn) were labeled by tetramethylrhodamine-dextran in a 7 days postfertilization (dpf)Tg(zic1:Gal4VP16/UAS:GFP) embryo. PLLg, posterior lateral line ganglia. A: Lateral view, dorsal is to the top, anterior is to the left. The central nerves of the posterior lateral line enter the hindbrain, then bifurcate and extend longitudinally in the dorsal hindbrain (Alexandre and Ghysen,[1999]), where the green fluorescent protein-positive (GFP(+)) neurons are abundant. B: Coronal section, dorsal is to the top, lateral is to the left. Most of the GFP(+) neurons are located dorsomedially to the axon terminals of the PLLn. Many GFP(+) neuropiles extending toward the terminals of the PLLn were observed (inset, arrowheads). This organization at the larval stage is similar to that of the adult zebrafish and goldfish (New et al.,[1996]; Wullimann et al.,[1996]). C: Schematic illustration of the connectivity between the octaval and octavolateral nuclei and torus semicircularis (TS) in zebrafish embryos (left) and in adult goldfish (right). In the dorsolateral hindbrain of zebrafish embryo (left), at least two distinct classes of neurons are distinguished by the cell locations, molecular profiles, and projection patterns. The commissural axons of the zn-5(+)Lhx2/9(+) neurons (red, filled ovals) form the lateral longitudinal fascicle and project to the midbrain TS. They likely contribute to both the auditory-related and lateral line-related octaval and octavolateral nuclei. It remains to be determined, however, whether they are already segregated in embryos or segregated later at an adult stage (compare with the illustration of adult goldfish on the right). The commissural axons of the Gad1/2(+) neurons (red, open ovals) project to the contralateral hindbrain. For simplicity, projections only from the left side are shown. The bHLH factor Atoh1a is specifically required for development of the zn-5(+)Lhx2/9(+) neurons but not for development of the Gad1/2(+) neurons. In adult goldfish (right), the ascending projection neurons (yellow, filled oval and solid line) in the medial octavolateral nucleus (MON) provide lateral line information to the lateral region of the midbrain TS (yellow; McCormick and Hernandez,[1996]; New et al.,[1996]). There is also a projection to the contralateral MON (yellow, open oval and dotted line; New et al.,[1996]). It is unknown whether distinct neuronal populations project separately to the TS or contralateral MON, or the same neuronal population projects to both the TS and contralateral MON (New et al.,[1996]). The ascending projection neurons (blue, filled oval and solid line) in the descending octaval nucleus (DON) provide auditory information to the medial region of the TS (blue) in goldfish (McCormick and Hernandez,[1996]; Yamamoto and Ito,[2005]). Similarly to the MON, there is also a projection to the contralateral DON (blue, open oval and dotted line; Yamamoto and Ito,[2005]). In other fish species such as carp and catfish, the anterior octaval nucleus (AON) also provides auditory information to the medial region of the TS (Echteler,[1984]; Yamamoto and Ito,[2005]). In goldfish, this nucleus seems to provide few, if any, projections to the TS (McCormick and Hernandez,[1996]; Yamamoto and Ito,[2005]). UAS, upstream activating sequences. Scale bar = 50 μm. |