- Title
-
phospholipase C, beta 3 is required for Endothelin1 regulation of pharyngeal arch patterning in zebrafish
- Authors
- Walker, M.B., Miller, C.T., Swartz, M.E., Eberhart, J.K., and Kimmel, C.B.
- Source
- Full text @ Dev. Biol.
|
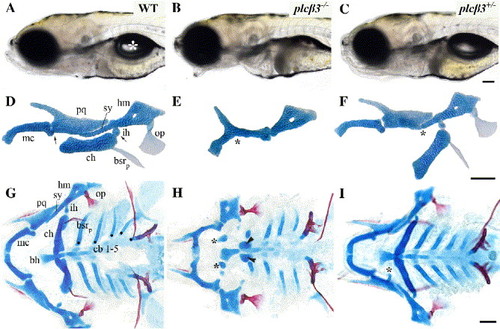
plcβ3 mutants have defects in facial skeletal patterning. (A--C) Lateral views of live wild-type, plcβ3 homozygous, and plcβ3 heterozygous larvae at 6 dpf. plcβ3 homozygotes have an open-mouth phenotype and fail to form a swimbladder (white asterisk in panel A). (D--F) Flat mounts of wild-type, plcβ3 homozygous, and plcβ3 heterozygous larvae Alcian-green-stained mandibular and hyoid cartilages at 6 dpf. Homozygotes have fusions at the joint region between the palatoquadrate and Meckel's cartilage of the mandibular arch and strong reductions in the symplectic and ceratohyal cartilages of the hyoid arch. Meckel's cartilage is less severely reduced in length and the palatoquadrate is reduced and/or misshaped. Wild-type D/V joint regions are indicated with arrows in panel D. Fusions at joint regions in plcβ3 homozygotes are indicated with asterisk in panel E. Reductions in symplectic cartilage in plcβ3 heterozygotes are indicated with asterisk in panel F. Cartilages are labeled as followed: pq (palatoquadrate), mc (Meckel's cartilage), hm (hyomandibula), ch (ceratohyal), sy (symplectic), and ih (interhyal). Two bones of hyoid arch are also lightly stained with Alcian green: op (opercle), bsrp (branchiostegal ray posterior). The opercle is frequently reduced or expanded in plcβ3 heterozygotes, while branchiostegal ray posterior is frequently absent. (G--I) Flatmounts of wild-type, plcβ3 homozygous, and plcβ3 heterozygous larvae doubly stained with Alcian blue and Alizarin red for cartilage and bone, respectively. In homozygotes ectopic cartilage nodules are frequently located near basihyal (bh) cartilage (asterisks in panel H). Ceratohyal cartilages are also +-frequently reduced to nubbins (arrowheads in panel H). Anterior ceratobranchials (cb) are typically reduced in length. Ceratobranchials are indicated with dots in panel G. In heterozygotes ectopic cartilage nodules frequently extend from the palatoquadrate cartilage (asterisk in panel I). Scale bars: 50 μm. |
|
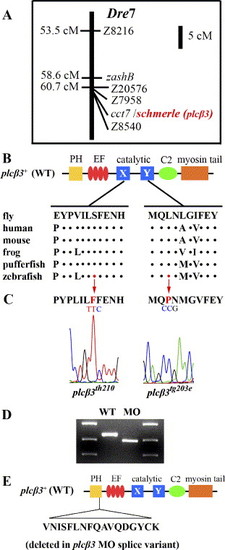
Segregation and sequence analysis of two plcβ3 alleles and plcβ3 MO splice variant. (A) schmerle (plcβ3) maps to Danio rerio chromosome 7 between microsatellites Z7958 and Z8540. (B) Schematic of protein domains in plcβ3+(wt) and lesions in plcβ3tg203e and plcβ3th210. Sequence around lesion sites is aligned with other vertebrate Plcβ3 and with Plc in fly. Dots indicate sequence identity. Abbreviations: PH (pleckstrin homology), EF (EF hand), C2 (protein kinase C conserved region 2). (C) Sequence chromatograms of plcβ3tg203eand plcβ3th210 around lesion sites in plcβ3. In plcβ3tg203e a T-to-C mutation results in the transformation of a conserved serine to a phenylalanine in the X domain of the catalytic domain. In plcβ3th210 a C-to-T mutation results in the transformation of a conserved leucine to proline in the Y domain of the catalytic domain. (D) RT-PCR analysis of plcβ3 mRNA structure in wild-type and splice MO-injected embryos. plcβ3 splice-blocking MO is complementary to exon 4 splice donor site. Primers designed to exon1 and exon5 were used in RT-PCR of uninjected and plcβ3 splice-blocking MO injected embryos at 1 dpf (5′CCGTTGTTACACTGAAGGT3′/5′GCTTTGAGTAAGAAGGTGTTG3′). (E) cDNA sequence comparison reveals that plcβ3 splice-blocking MO variant results from aberrant splicing to a cryptic splice site locate 51 bases 5′ of the normal exon4 splice donor. The plcβ3 MO splice variant results in the deletion of 17 amino acids within the PH domain of plcβ3. EXPRESSION / LABELING:
|
|
A splice-blocking MO partially rescues plcβ3 homozygotes. (A–D) Flatmounts of 5–6 dpf wild-type and plcβ3 homozygotes pharyngeal tissues doubly stained with Alcian blue and Alizarin red for cartilage and bone, respectively. (A) Uninjected wild type. (B) Injection of plcβ3 splice-blocking MO into wild-type yields only mild phenotypes. Arrow indicates reduction of symplectic cartilage. (C) plcβ3 homozygotes have a loss of jaw joints, misshaped palatoquadrate cartilage, and a strong reduction of symplectic (arrows) and ceratohyal cartilages (arrowheads). (D) Injection of plcβ3 splice-blocking MO into plcβ3 heterozygotes partially rescues cartilage defects. Arrows indicate reduction of symplectic cartilage. Asterisks indicate ectopic cartilage nodule extending from palatoquadrate. Jaw joints and ceratohyal cartilages are strongly rescued. Scale bar: 50 μm. |
|
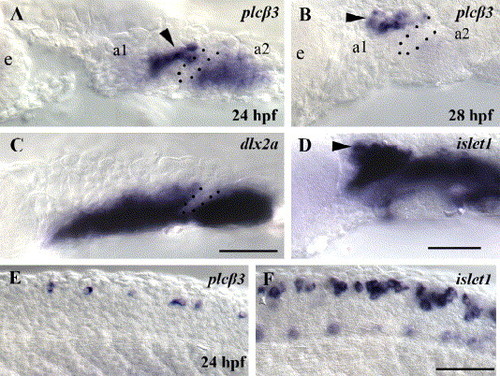
plcβ3 is expressed in the pharyngeal arches and in neuronal cells. Lateral views of in situ hybridizations in wild-type embryos at 24 (A, C, E, F) and 28 hpf (B, D). Anterior is to the left. At 24 and 28 hpf, plcβ3 is expressed in a first arch domain adjacent to the first pouch (A, B), similar to a subset of the islet1 trigeminal ganglion domain (D). At 24 hpf, plcβ3 is also expressed in the second arch (A), similar to the dlx2a neural crest domain (C). At 28 hpf, plcβ3 is only weakly expressed in the second arch neural crest domain (B). Lateral trunk views at the same axial level of plcβ3 (E) and islet1 (F) RNA in situ hybridizations with wild-type embryos at 24 hpf. plcβ3 is expressed in a subset of cells at appropriate positions to be Rohon–Beard spinal sensory neurons. a1 and a2 label pharyngeal arch 1 and 2. Dotted lines label pharyngeal pouch 1. Arrowheads label cells at position of trigeminal ganglion domain. Scale bar: 50 μm. EXPRESSION / LABELING:
|
|
plcβ3 is required for edn1-dependent Dlx gene expression. Lateral views of in situ hybridizations in wild-type and plcβ3 homozygotes at 30 hpf (A–H) and 36 hpf (I–P). (A, E, I, M) dlx2a, (B, F, J, N) dlx5a, (C, G, K, O) dlx6a, and (D, H, L, P) dlx3b. At 30 hpf, expression of dlx2a is moderately affected in the most ventral domain in plcβ3 homozygotes (E), and at 36 hpf dlx2a expression is clearly reduced in a ventral arch domain in both the first and second arches in plcβ3 homozygotes (M). At 30 hpf, the expressions of dlx3b, dlx5a, and dlx6a are strongly reduced in both the first and second arches in plcβ3 homozygotes (F, G, H). At 36 hpf, expressions of dlx6a and dlx3b are still strongly reduced in plcβ3 homozygotes (O, P). At 36 hpf, expression of dlx5a has recovered in a dorsal arch domain but is still strongly reduced in intermediate and ventral arch domains (N). a1 and a2 label pharyngeal arch 1 and 2. Dotted lines label pharyngeal pouches 1 and 2 in panels A–P and bottom of arch 1 and 2 in panels F, N, G, O, H, and P. Scale bar: 50 μm. |
|
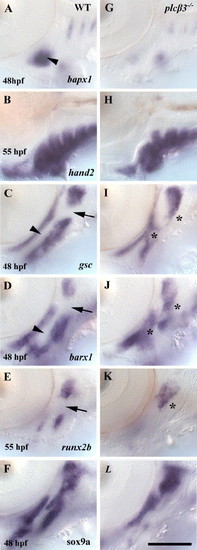
Intermediate and ventral pharyngeal arch domains are misspecified in plcβ3 mutants. Lateral views of in situ hybridizations in wild-type and plcβ3 homozygotes. (A, G) At 48 hpf, bapx1 expression in a first arch intermediate domain is strongly reduced in plcβ3 homozygotes. (B, H) At 55 hpf, hand2 expression is at near wild-type levels in plcβ3 homozygotes. (C, I) At 48 hpf, gsc is ectopically expressed in first and second arch intermediate domains and strongly reduced in a second arch ventral domain in plcβ3 homozygotes. (D, J) At 48 hpf, barx1 expression in the first and second arches is ectopically expressed in intermediate domains and strongly reduced in ventral domains in plcβ3 homozygotes. (E, K) At 55 hpf, runx2b is ectopically expressed in a second arch intermediate domain and strongly reduced in a second arch ventral domain in plcβ3 homozygotes. (F, L) At 48 hpf, sox9a expression is strongly reduced in ventral domains of the first and second arches in plcβ3 homozygotes. Arrowheads indicate first arch intermediate domain in wild-type embryos. Arrows indicate second arch intermediate domains in wild-type embryos. Asterisks indicate ectopic expression in intermediate arch domains in plcβ3 homozygotes. a1 and a2 label pharyngeal arch 1 and 2. Scale bar: 50 μm. |
|
plcβ3 is required in cranial neural crest cells for pharyngeal arch patterning. (A--C) Projections of confocal z-stacks, including fluorescent and bright-field channels, of lateral and orthogonal views of live 36 hpf mosaic host embryos. (A) Cells from the animal pole of Alexa568-labeled sphere staged (6 hpf) wild-type fli:GFP donors were transplanted unilaterally into (A) the neural crest domain, (B) the surface ectoderm domain, and (C) the endoderm domain of plcβ3 homozygous hosts. Embryos were screened at 30–36 hpf to determine contribution of donor cells to pharyngeal arches and again at 6 dpf to assess contribution of donor cells to cartilages. Dots indicate bottom of the first pharyngeal pouch. Green florescence indicates presence of fli-positive donor neural crest in transplants in panel A and absence of green florescence indicates absence of neural crest in transplants in panels B and C. (D--F) Embryos imaged in panels A–C were subsequently fixed and doubly stained for both cartilage (Alcian blue) and bone (Alizarin red). Non-transplant sides of hosts were used as negative controls for rescue. As brain tissues frequently accompany large neural crest transplants, we also analyzed transplants containing only brain tissue and determined they did not rescue (n = 5, data not shown). Scale bars: 50 μm. |
|
plcβ3 is required in cranial neural crest cells for ventral pharyngeal muscle patterning. Flat mount in Fig. 7D was subsequently imaged with DIC to show muscle patterning. On transplant side wild-type neural crest cells partially rescue patterning of several ventral arch muscles. On non-transplant side, muscle fibers are largely disorganized. Muscles are abbreviated as follows: intermandibularis posterior (imp), interhyal (ih), hypohyal (hh) ((Schilling and Kimmel, 1997). Asterisks (*) indicate disorganized muscle fibers on non-transplant side of plcβ3 homozygous host. Scale bar: 50 μm. |
|
plcβ3-/- neural crest cells sort from wild-type neural crest cells in mosaic embryos. (A–F) Confocal fluorescent stacks of live 6 dpf mosaic host embryos. (A, C, E) Wild-type Alexa568-labeled neural crest cells were transplanted into wild-type fli:GFP hosts at sphere stage (6 hpf). Wild-type donor cells contributed to (A) hyomandibular (hm), (C) symplectic (sy), (E) interhyal (ih), and ceratohyal (ch) cartilages. (B, D, F) plcβ3 homozygous Alexa568-labeled neural crest cells were transplanted into wild-type fli:GFP hosts at sphere stage (6 hpf). plcβ3 mutant donor cells contributed significantly to (B) hyomandibular, but not to (D) symplectic, (F) interhyal, or ceratohyal cartilages. (G, H) Schematic of cell sorting observed in mosaic transplants. (G) Wild-type to wild-type transplants did not sort, whereas (H) mutant to wild-type transplants sorted. Red indicates donor and green indicates hosts. Scale bar: 50 μm. |

Unillustrated author statements |
Reprinted from Developmental Biology, 304(1), Walker, M.B., Miller, C.T., Swartz, M.E., Eberhart, J.K., and Kimmel, C.B., phospholipase C, beta 3 is required for Endothelin1 regulation of pharyngeal arch patterning in zebrafish, 194-207, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.