- Title
-
Fgf signaling negatively regulates Nodal-dependent endoderm induction in zebrafish
- Authors
- Mizoguchi, T., Izawa, T., Kuroiwa, A., and Kikuchi, Y.
- Source
- Full text @ Dev. Biol.
|
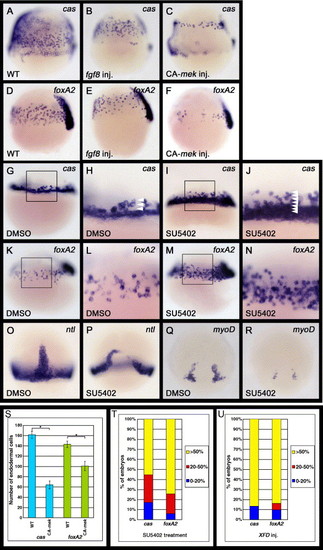
Fgf signaling can regulate the formation of endoderm and mesoderm. (A–F) Zebrafish embryos injected with fgf8 or CA-mek mRNA were examined for cas or foxA2 expression at the 60% epiboly stage (7 h postfertilization [hpf]). Lateral views, dorsal to the right. The number of cas- or foxA2-expressing cells is decreased in embryos in which Fgf signaling is activated. (G–N) Embryos treated with DMSO or SU5402 were examined for cas or foxA2 expression at either the 40% (5 hpf) or 60% epiboly stage (7 hpf), respectively. DMSO, SU5402: oep mRNA was injected into WT embryos at the one cell stage before treatment with DMSO or SU5402. (G–J) Lateral views, anterior to the top. (H and J) Enlarged views of the boxed area in panels G and I, respectively. Arrowheads in panels H and J indicate the number of cas-expressing cells from the margin. SU5402 treatment significantly increases the number of cas-expressing endodermal cells around the marginal domain. (K–N) Lateral views, dorsal to the right. (L and N) Enlarged views of the boxed areas in panels K and M, respectively. The number of foxA2-expressing endodermal cells is also significantly increased by SU5402 treatment. (O–R) Embryos treated with SU5402 were examined for expression of the pan-mesodermal marker ntl and adaxial maker myoD at the 80% epiboly stage (8. 3 hpf). The ntl and myoD expression levels were reduced by SU5402 treatment at the late stages of gastrulation. (S) Effects of the activation of Fgf signaling on the number of cas- or foxA2-expressing endodermal cells. The number of cas- or foxA2-expressing endodermal cells was counted at the 60% epiboly stage on both the left and right lateral sides of the embryos, which we determined to be representative of whole embryos. The embryos analyzed were cas-expressing endodermal cells in WT (n = 37) and CA-mek-injected (n = 45) embryos; foxA2-expressing endodermal cells in WT (n = 52); and CA-mek-injected (n = 38) embryos. Error bars represent the standard error. *P < 0.0005, Student′s t test. (T, U) Effects of the inhibition of Fgf signaling by SU5402 treatment (T) or XFD overexpression (U) upon the number of cas- or foxA2-expressing endodermal cells. oep mRNA was injected into WT embryos at the one cell stage before treatment with SU5402 (T) and was co-injected with XFD mRNA at the one cell stage (U). Zebrafish embryos at the 60% epiboly stage (7 hpf) were scored as demonstrating higher cas or foxA2 expression levels if they exhibited a greater than 50% (yellow), 20–50% (red) or 0–20% (blue) increase in endodermal cells. However, because SU5402 treatment or XFD overexpression greatly increases the number of cas- and foxA2-expressing endodermal cells, it is difficult to determine their exact number. The embryos analyzed were (T) cas-expressing endodermal cells in DMSO-treated (n = 37) and SU5402-treated (n = 48) embryos; foxA2-expressing endodermal cells in DMSO-treated (n = 52) and SU5402-treated (n = 51) embryos: (U) cas-expressing endodermal cells in WT (n = 28) and XFD-injected (n = 30) embryos; foxA2-expressing endodermal cells in WT (n = 33) and XFD-injected (n = 31) embryos. EXPRESSION / LABELING:
|
|
SU5402 treatment does not affect the expression of either Nodal or Nodal antagonists. (A–H) All images are lateral views with anterior to the top. These are magnified views of zebrafish embryos around the marginal region at the 40% epiboly stage (5 hpf). DMSO, SU5402: oep mRNA was injected into WT embryos, and these injected embryos were subsequently treated with either DMSO or SU5402 from the one cell stage to the 40% epiboly stage. (B, D, F, H) The expression of Nodal genes (cyc and sqt) and Nodal antagonists (lefty1 and lefty2) are not affected by SU5402 treatment. |
|
Fgf signaling does not affect bon and fau/gata5 expression. (A–L) All images are lateral views with anterior to the top. These are magnified views of zebrafish embryos around the marginal region at the 40% epiboly stage (5 hpf). (A–B, E–F, I–J) DMSO, SU5402: oep mRNA was injected into WT embryos, and these injected embryos were subsequently treated with either DMSO or SU5402 from the one cell stage to the 40% epiboly stage. Neither the bon nor the fau/gata5 expression levels are affected, whereas mezzo expression is barely detectable, in SU5402-treated embryos. (C–D, G–H, K–L) Overexpression of CA-mek mRNA in WT embryos does not alter the bon or fau/gata5 expression levels, whereas mezzo expression around the marginal region is increased in the CA-mek mRNA-injected embryos. |
|
Inhibition of Fgf signaling increases the number of cas- and foxA2-expressing endodermal cells in bon and fau mutants. (A–J) Lateral views of zebrafish embryos with dorsal to the right. bon or fau heterozygote intercrosses were injected with oep and XFD mRNAs at the 1- to 4-cell stage and were examined for cas or foxA2 expression at the 80% epiboly stage (8. 3 hpf). Inhibition of Fgf signaling can increase the number of cas- and foxA2-expressing endodermal cells in both bon and fau mutant embryos. (K) Effects of the inhibition of Fgf signaling on the number of cas- or foxA2-expressing endodermal cells in bon mutant embryos. The number of cas- or foxA2-expressing endodermal cells was counted at the 80% epiboly stage on both the left and right lateral sides of the embryos, which we determined to be representative of whole embryos. The embryos analyzed were cas-expressing endodermal cells in oep-injected WT (n = 21) and oep-injected (n = 15) and oep,XFD-injected (n = 20) bon mutant embryos; foxA2-expressing endodermal cells in oep-injected WT (n = 24) and oep-injected (n = 12) and oep,XFD-injected (n = 23) bon mutant embryos. Error bars represent the standard error. *P < 0.0001, Student′s t test. (L) Effects of the inhibition of Fgf signaling upon the number of cas- or foxA2-expressing endodermal cells in fau mutant embryos. Zebrafish embryos at the 80% epiboly stage (8. 3 hpf) were scored as demonstrating higher cas or foxA2 expression levels if they exhibited a greater than 50% (yellow), 20–50% (red) or 0–20% (blue) increase in endodermal cells. However, because oep,XFD mRNAs injection greatly increases the number of cas- and foxA2-expressing endodermal cells, it is difficult to determine their exact number. The embryos analyzed were cas-expressing endodermal cells in oep-injected (n = 14) and oep,XFD-injected (n = 17) fau mutant embryos; foxA2-expressing endodermal cells in oep-injected (n = 12) and oep,XFD-injected (n = 15) fau mutant embryos. |
|
Either bon or fau/gata5 overexpression can increase the number of cas-expressing endodermal cells in Fgf signaling-inhibited embryos. (A–C) All images are lateral views with anterior to the top. These are magnified views of zebrafish embryos around the marginal region at the 40% epiboly stage (5 hpf). Arrowheads indicate the number of cas-expressing cells from the margin. (A) Overexpression of XFD and oep in WT embryos increases the number of cas-expressing endodermal cells to up to 8 cell diameters from the margin. (B) The cas-expressing endodermal cells were observed to be increased to 11 cell diameters from the margin by co-injection of XFD and oep with bon into WT embryos. (C) Most of the marginal cells within 11 cell diameters from the margin express cas following co-injection of XFD and oep with fau/gata5 into WT embryos. |
|
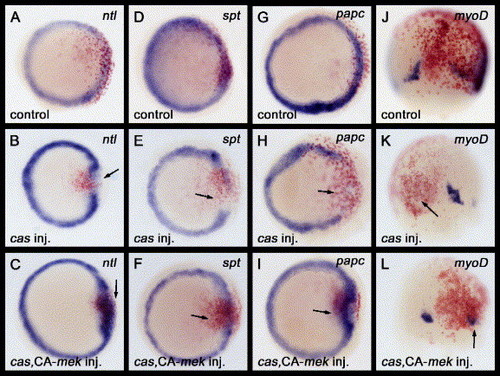
The activation of Fgf signaling can restore the expression of mesodermal-specific genes that are down-regulated by cas misexpression. (A–L) Either cas mRNA, or a combination of cas with CA-mek mRNAs, were injected into a marginal blastomere of a 16-cell stage zebrafish embryo. Embryos injected with mRNA(s) were examined for ntl, spt or papc expression at the 50% epiboly stage (5. 3 hpf) and myoD expression at the 80% epiboly stage (8. 3 hpf). Nuclear lacZ (nlacZ) mRNA was used as a lineage tracer. Red staining by β-galactosidase activity indicates the progeny of injected cells. The progeny of marginal blastomeres gives rise to the marginal clones of the cells. (A–I) All images are of animal pole views. (J–L) Dorsal views with anterior to the top. (A, D, G, J) The nlacZ mRNA injections did not disrupt the ntl, spt, papc or myoD expression profile. (B, E, H, K) The expression of the mesodermal genes ntl, spt, papc or myoD was down-regulated by cas mRNA misexpression (arrows). (C, F, I, L) The down-regulation of the mesodermal genes ntl, spt, papc or myoD by cas mRNA misexpression was restored by co-injection with CA-mek mRNA (arrows). EXPRESSION / LABELING:
|
|
A model for endoderm and mesoderm formation in zebrafish. In late blastula stage zebrafish embryos, there are two steps in endoderm and mesoderm induction. (A) Zygotic oep expression is induced by Fgf signaling. The Nodal-related proteins Cyc and Sqt act through TGF-β type receptors. Oep is maternally supplied and it is an essential co-receptor for Nodal signaling (Gritsman et al., 1999). Nodal signaling induces fgf and this Fgf signaling functions to induce zygotic oep expression (Mathieu et al., 2004). Thus, the combination of Nodal and Fgf signaling is necessary for both endoderm and mesoderm induction. (B) Induction of endoderm and mesoderm by Nodal and Fgf signaling. Nodal signaling activates bon and fau/gata5 expression. Bon, Fau/Gata5 and Eomes cooperatively regulate the expression of cas (Bjornson et al., 2005). *Eomes; eomes is maternally supplied to the zygote. Cas activates sox17 and foxA2 and represses mesodermal genes to determine endodermal fates. On the other hand, both Fgf and Nodal signaling synergistically activate mesodermal genes, such as ntl, spt, papc and myoD. Fgf and MAPK signaling also negatively regulate cas expression and Cas function to determine the mesodermal fate. |
Reprinted from Developmental Biology, 300(2), Mizoguchi, T., Izawa, T., Kuroiwa, A., and Kikuchi, Y., Fgf signaling negatively regulates Nodal-dependent endoderm induction in zebrafish, 612-622, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.