- Title
-
Zebrafish Foxd3 is required for development of a subset of neural crest derivatives
- Authors
- Lister, J.A., Cooper, C., Nguyen, K., Modrell, M., Grant, K., and Raible, D.W.
- Source
- Full text @ Dev. Biol.
|
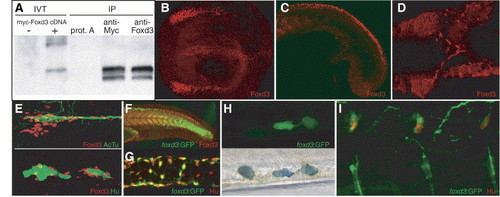
Expression of Foxd3 protein during zebrafish embryogenesis. (A) Western blot of immunoprecipitated Foxd3 proteins. Left lanes show biotinylated in vitro translation (IVT) reactions with or without cDNA. Right lanes show immunoprecipitation (IP) of in vitro translation reactions with anti-Myc or anti-Foxd3 antibody compared to control where antibody was omitted (prot. A). Biotinylated protein was detected using avidin–HRP. (B–E) Confocal images of Foxd3 antibody staining. (B) Expression in neural crest and floor plate of a 6-somite embryo. Dorsal view, anterior left. (C) Expression in caudal neural crest and somites of an 18 hpf embryo. (D) Expression at 36 hpf in migrating vagal neural crest cells, ventral view with yolk removed. (E) Expression in presumptive glial cells along the posterior lateral line nerve and ganglia. Top panel: red—Foxd3, green—acetylated tubulin. Bottom panel: red—Foxd3, green—Hu. (F) Comparison of Foxd3 immunoreactivity with expression of GFP in the foxd3:GFP transgenic line at 24 hpf. GFP persists in rostral neural crest cells where Foxd3 protein is no longer detectable. (G) Expression of GFP (detected with α-GFP antibody) in a subset of enteric neurons in the dissected intestine of a 96 hpf foxd3:GFP larva. Green—GFP, red—Hu. (H) Expression of GFP in a subset of tail iridophores of a live 72 hpf foxd3:GFP larva. Note GFP-negative iridophore to the left of two GFP-expressing iridophores (iridophore pigments appear dark in this transmitted light image, bottom panel). (I) Confocal image showing expression of GFP (detected with α-GFP antibody) in dorsal root ganglia neurons of a 96 hpf foxd3:GFP larva. Green—GFP, red—Hu. EXPRESSION / LABELING:
|
|
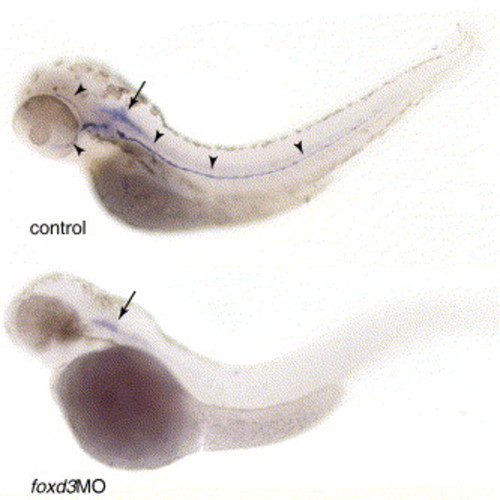
Morpholino knockdown of Foxd3 expression. Gross loss of function phenotype: uninjected control larva (top panel) and injected with foxd3 morpholino (bottom panel). Black arrowheads indicate lower jaw, reduced in size in the foxd3 MO-injected larva. White arrowheads indicate iridophores that are reduced in the Foxd3 morpholino-injected larva. |
|
Morpholino knockdown effect on neural crest markers. (A–C) 6 somite stage. Viewed dorsally, anterior to the left. (A) Foxd3 protein; in situ hybridization for (B) snai1b, (C) sox10. (D–F) 18 somite stage, lateral views. In situ hybridization for (D) crestin, (E) snai1b, (F) sox10. snai1b expression is greater in morpholino-injected embryos than in controls (arrowheads). EXPRESSION / LABELING:
|
|
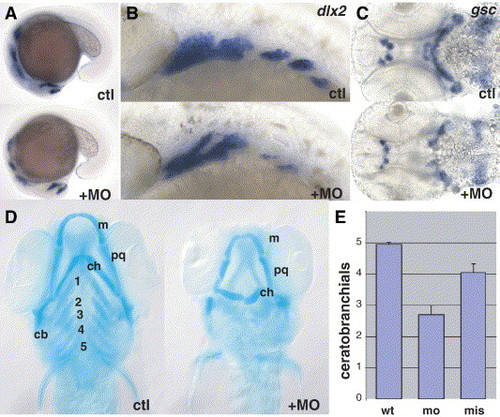
MO knockdown: jaw cartilage. (A) Slight reduction in dlx2 staining at 18 hpf (lateral view). (B) Reduction in dlx2 is prominent in posterior arches at 27 hpf. (C) Reduction in gsc staining at 48 hpf, ventral view. (D) Reduced cartilage elements in 4 dpf larvae revealed by Alcian blue staining, ventral view. Plainly visible jaw defects were observed in 36% of Alcian blue-stained embryos (n = 14) injected with MO at 1 mg/ml, and 82% of embryos (n = 28) injected at 2 mg/ml, compared with 0% (n = 19) injected with buffer alone. m, Meckel′s cartilage; pq, palatoquadrate; ch, ceratohyal; cb, ceratobranchial; bb, basibranchial. (E) Ceratobranchial elements are significantly reduced after morpholino injection (n = 30) when compared to mismatch control (P = 0.002). A slight but consistent reduction is also seen after mismatch morpholino (n = 26) compared to uninjected controls (n = 22). EXPRESSION / LABELING:
|
|
MO knockdown: pigment cell differentiation markers, in situ hybridizations. (A) Histogram of tail iridophore counts. Both MOs were injected at 2 mg/ml. The mean number of tail iridophores per embryo ± SEM are depicted for uninjected (n = 60), morpholino injected (n = 107), and mismatch morpholino injected (n = 83). (B) Presumptive iridophores expressing ednrb1 (endothelin receptor b1) are absent from Foxd3 morpholino-injected embryos. (C) Melanoblast marker dct (dopachrome tautomerase) is unaffected. (D) Xanthoblast marker xdh (xanthine dehydrogenase) is slightly reduced in the trunk. EXPRESSION / LABELING:
|
|
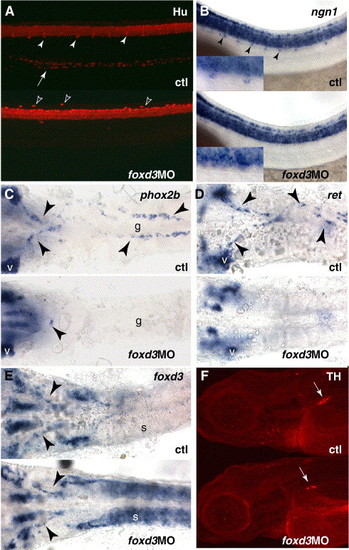
Morpholino knockdown and loss of peripheral neuronal markers. (A) Loss of Hu immunoreactivity from the dorsal root ganglia (arrowheads) and gut (arrow) of embryos injected with foxd3 morpholino, lateral view of trunk at 72 hpf. Morpholino-injected embryos frequently display ectopic Hu-positive cells dorsally (black arrowheads). (B) ngn1 in situ hybridization at 36 hpf, showing absence of DRG precursors (arrowheads, see inset). (C–E) In situ hybridizations at 36 hpf, ventral views with yolk removed, indicating migrating vagal neural crest cells and enteric neuron precursors in the gut tube (arrowheads). V, vagal ganglia; g, gut tube; s, somites. (C) phox2b. (D) ret. (E) foxd3. (F) tyrosine hydroxylase staining of sympathetic ganglia (arrow) that is reduced in morpholino-injected embryos at 96 hpf, lateral views. EXPRESSION / LABELING:
|
|
Morpholino knockdown and loss of peripheral glia. In situ hybridizations of 72 hpf larvae showing loss of myelin basic protein (mbp) expression in the lateral line (arrowheads). Note that expression in the CNS (arrow) is not affected. EXPRESSION / LABELING:
|
|
Cell autonomy of foxd3 function. (A, B) foxd3:GFP donor cells into Foxd3 morphant. Rescue of lateral line glia (green; arrowheads) in two embryos. Arrows indicate donor-derived neuromasts (red). (C) Uninjected donor into Foxd3 morpholino-injected host, immunostained for Hu (green). Arrowheads indicate rescued DRG neurons, derived from donor (red). |
|
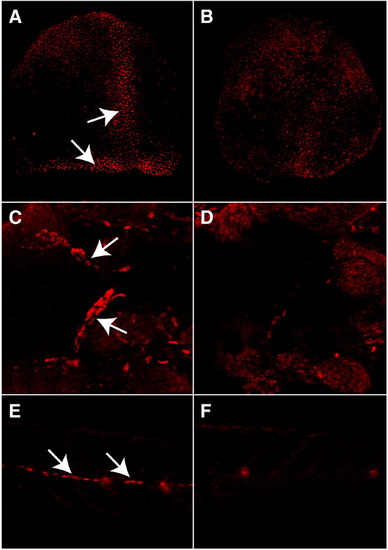
Antisense morpholino oligonucleotides block Foxd3 protein expression at all stages examined. Confocal images of immunocytochemistry of control (A, C, E) and morpholino-injected (B, D, F) embryos. (A, B) Dorsal view of 80% epiboly stage embryos. Arrows in panel (A) show Foxd3 expression in the dorsal margin and involuting axial mesoderm. (C, D) Ventral view of embryos at 36hpf. The yolk has been removed to image the anterior gut tube. Arrows in panel (C) show migrating neural crest-derived enteric precursors. (E, F) Lateral view of tail segments of embryos at 72 hpf. Arrows in panel (E) show lateral line nerve glial cells along the horizontal myoseptum. EXPRESSION / LABELING:
|
|
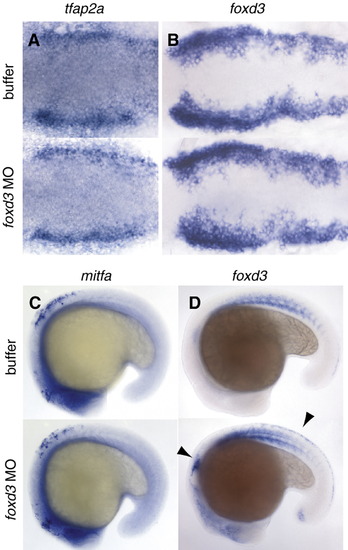
Morpholino knockdown effect on neural crest markers. A-B, 6 somite stage. Viewed dorsally, anterior to the left. In situ hybridization for A) tfap2, B) foxd3. C-D) 18 somite stage, lateral views. In situ hybridization for C) mitfa, D) foxd3. Note expression of foxd3 is greater in morpholino-injected embryos than in controls (arrowheads). EXPRESSION / LABELING:
|
|
Morpholino knockdown and loss of peripheral glial markers. In situ hybridizations of 72 hpf larvae showing loss of marker expression in the lateral line. (A) oct6. (B) krox20. Note that expression in the CNS is not affected. EXPRESSION / LABELING:
|
|
Dorsalization of embryos as a result of early foxd3 overexpression. Live embryos area shown at 24 h post-fertilization. (A) Uninjected control embryo. (B) Embryo injected with 100pg myc-foxd3 mRNA. (C) Embryo injected with 100pg myc-foxd3mut mRNA. |
Reprinted from Developmental Biology, 290(1), Lister, J.A., Cooper, C., Nguyen, K., Modrell, M., Grant, K., and Raible, D.W., Zebrafish Foxd3 is required for development of a subset of neural crest derivatives, 92-104, Copyright (2006) with permission from Elsevier. Full text @ Dev. Biol.