- Title
-
Zebrafish vps33b, an ortholog of the gene responsible for human arthrogryposis-renal dysfunction-cholestasis syndrome, regulates biliary development downstream of the onecut transcription factor hnf6
- Authors
- Matthews, R.P., Plumb-Rudewiez, N., Lorent, K., Gissen, P., Johnson, C.A., Lemaigre, F., and Pack, M.
- Source
- Full text @ Development
|
vps33b expression in zebrafish embryos and larvae. (A-I) Whole-mount RNA in situ hybridization. Sphere stage (A), 10 somite (B) and 24 hpf (C) embryos show diffuse vps33b expression. (D) vps33b expression at 48 hpf is evident in the developing liver (black arrow) and proximal intestine (blue arrow). (E) Lateral view of a 72 hpf larva showing vps33b expression in the liver (black arrow) and proximal intestine (blue arrow). Weak pancreas expression (white arrow) is also evident. (F,G) High-power lateral views of 4 dpf larvae processed for vps33b and ceruloplasmin whole-mount RNA in situ hybridization. Liver (black arrow) demonstrates a reticular pattern of vps33b expression (black arrow) (F) compared with a homogeneous pattern of ceruloplasmin expression (G). Intestinal vps33b expression is also evident (blue arrow, F). (H) Higher power view of liver depicted in F. (I) Enhanced view of H outlining putative ducts. (J,K) Histological cross-sections of a 4 dpf larva processed for vps33b (J) and ceruloplasmin (K) whole-mount RNA in situ hybridization. These panels show punctate regions of vps33b expression in presumptive bile ducts (green arrowheads) and a small number of hepatocytes (red arrowheads). Biliary epithelial cell size and cytoplasm in these panels are comparable with biliary epithelial cell ultrastructure (Fig. 5) (Lorent et al., 2004; Matthews et al., 2004). |
|
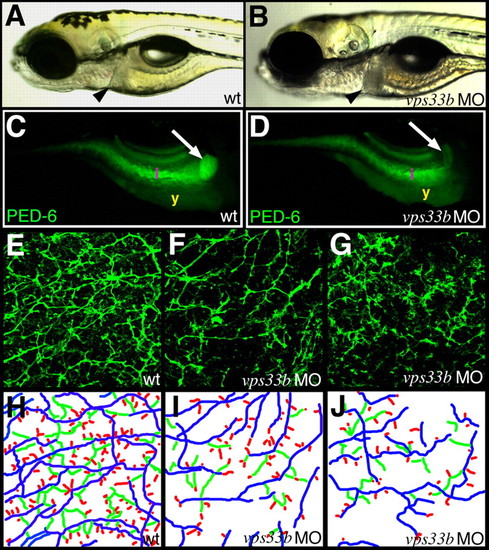
vps33b knockdown disrupts zebrafish intrahepatic biliary development. (A,B) Left lateral views of 5 dpf wild-type (A) and vps33b (B) morpholino-injected larvae. Liver size (black arrowheads) is comparable in these larvae. (C,D) Right lateral fluorescent images of 6 dpf wild-type (C) and vps33b morpholino injected larvae (D) following ingestion of the PED-6 lipid reporter. Gallbladder fluorescence (white arrow) is decreased in morpholino-injected (D) larva relative to wild-type larva (C). i, intestinal fluorescence; y, endogenous yolk fluorescence. (E-G) Confocal projections through the liver of 5 dpf wild-type (E) and vps33b (F,G) larvae processed for keratin 18 immunohistochemistry. There are fewer bile ducts in F than in E; ducts are sparse, with fewer interconnecting ducts and terminal ductules. (H-J) Colorized schematics of bile ducts from E-G. Long ducts depicted in blue, interconnecting ducts in green and terminal ductules in red. PHENOTYPE:
|
|
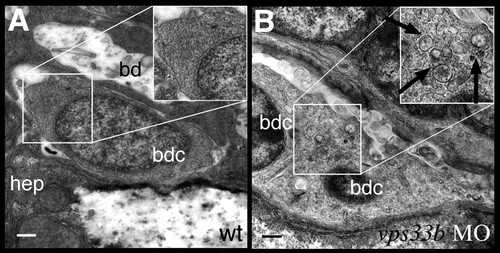
vps33b knockdown disrupts biliary ultrastructure. (A,B) Electron micrographs of biliary epithelial cells from 5 dpf wild-type (A) and vps33b morpholino injected (B) larvae. The wild-type biliary cell cytoplasm has a homogeneous appearance. (B) A small bile duct comprising two bile duct cells from a vps33b morpholino-injected larva. Cytoplasm appears heterogeneously with multiple vesicles (black arrows). hep, hepatoctye; bd, bile duct lumen; bdc, bile duct cell. PHENOTYPE:
|
|
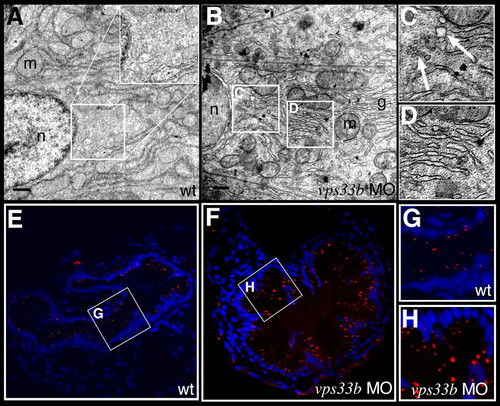
vps33b knockdown disrupts intestinal vesicle transport. (A-D) Electron micrographs of enterocytes from 5 dpf wild-type (A) and vps33b morpholino injected (B-D) larvae. The apical surface is to the right of each panel. Insets are at twice the magnification of the indicated regions of cytoplasm. No vesicles can be identified within the wild-type cell cytoplasm. (C) Multiple vesicles (arrows) are seen in the cytoplasm of vps33b-deficient enterocyte. Dilated stacks of Golgi cisternae are also evident within these cells (D). g, golgi; m, mitochondria; n, nucleus. (E-H) Histological cross-sections from the anterior intestine of 5 dpf wild-type (E,G) and vps33b morpholino-injected (F,H) larvae that have ingested the styryl dye AM1-43. Nuclei stained with DAPI. (G,H) Magnified views from E and F, respectively. There was a 1.8-fold increase in the number of fluorescent vesicles in the vps33b-deficient larvae (see Table 4). PHENOTYPE:
|
|
vps33b expression is regulated by hnf6/vhnf1. (A,B) vps33b expression in 3 dpf wild-type (cont) hnf6 morpholino-injected (A) and vhnf1mutant larvae (B) as determined by quantitative PCR. vps33b expression is reduced by 75% and 60%, respectively, in the hnf6 morpholino-injected larvae and vhnf1 mutants. Amplification levels normalized to wild type. (C-F) Whole-mount RNA in situ hybridization of 3 dpf wild-type (C,E) and hnf6 (D,F) morpholino-injected larvae using a ceruloplasmin (C,D) or a vps33b (E-F) riboprobe. Liver (black arrows) ceruloplasmin expression is unchanged in the hnf6 morpholino-injected larva, whereas decreased liver vps33b expression is evident. (G) Comparison of vps33b expression in 3 dpf wild-type larvae (cont) and in 3 dpf larvae injected with hnf6 morpholino (hnf6 MO), hnf6 morpholino and vhnf1mRNA, vhnf1 mRNA, or hnf6 mRNA. These experiments are normalized to control expression and show decreased vps33b expression (by 75%, as above) in hnf6-deficient larvae that is restored with co-injection of vhnf1 RNA. Microinjection of vhnf1 on its own increases vps33b expression (2.2×), as does hnf6 RNA to a lesser degree (1.3×). Error bars represent s.e.m. from six separate experiments. EXPRESSION / LABELING:
PHENOTYPE:
|