- Title
-
Zebrafish ftz-f1a (nuclear receptor 5a2) functions in skeletal muscle organization
- Authors
- Sheela, S.G., Lee, W.C., Lin, W.W., and Chung, B.C.
- Source
- Full text @ Dev. Biol.
|
Expression profile of ff1a during early somitogenesis. (A–E) In situ hybridization of ff1a, with anterior to the left. (A) ff1a transcripts are first localized as bilateral cells on either side of the notochord at 13 h, dorsal view. (B–F) Lateral view. (B) ff1a transcripts are most abundant at 18 h in anterior somites and in the neural tube (arrows). (C) After the mid-segmentation stage, ff1a expression is down-regulated from the anterior somites, but expression at the neural tube remains visible at 20 h. (D) ff1a expression is very weak even from posterior somites at 22 h. (E) When compared to myoD expression pattern (pink color), ff1a (blue) is expressed in the adaxial cells of newly formed somites and gradually migrates out to the periphery. The dotted line represents the position of the cross-sections shown in panels (G, H). (F) A cartoon drawing of panel (E). (G) Cross-sections of double in situ with ff1a (blue) and myoD (pink). (H) Cross-sections of double in situ with ff1a (black) and shh (pink). EXPRESSION / LABELING:
|
|
ff1a is expressed in the migrating slow muscle precursor cells. Cross-sections of 24-hpf embryos showing that the migration patterns of (A) ff1a-expressing and (B) F59 positive slow muscle cells are comparable. Dorsal to the top. Somite number counting from the most posterior end is referred to by Roman Numerals and may have 1 somite difference, i.e., Somite I for newly formed 1st and 2nd somites, IV for 3rd to 5th somites, VII for 6th to 8th somites, and so on. In sections of Somite I, the slow muscle precursor cells are located in the adaxial region. These ff1a-expressing cells migrate dorso-ventrally first as shown for Somites IV and VII and then migrate laterally (somites X and XIV). During lateral migration, ff1a transcripts start to be down-regulated (in Somites X and XIV). The pattern of slow muscle heavy chain detected by F59 staining is similar to that of ff1a expression, except that F59 staining remains strong in Somite XIV. EXPRESSION / LABELING:
|
|
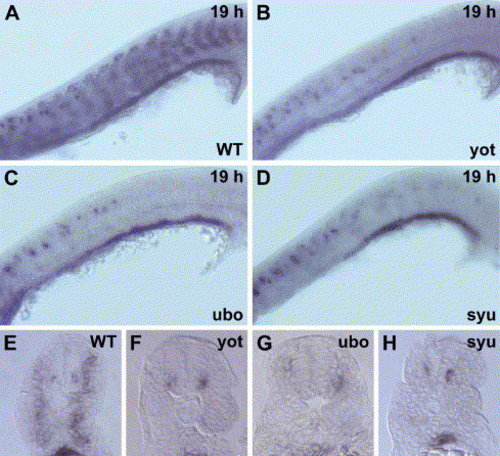
Disruption of ff1a expression in slow muscle mutants. (A–D) Lateral view with anterior towards the left, (E–H) cross-sections of anal somites. (A, E) At 19 hpf, ff1a expression in the slow muscle precursor (arrows) and endoderm of the wild-type embryo (WT) is evident. The expression of ff1a is abolished in the slow muscle cells of (B, F) yot and (C, G) ubo mutants, and very faint in (D, H) syu mutant (arrow). Instead, the signals are strong in the neurons as lines of dots and in the endoderm. EXPRESSION / LABELING:
|
|
Expression domain of ff1a in slow muscle is modulated by components of the Hedgehog signaling pathway. (A–F) Lateral view with the anterior to the left. (A) The expression domain of ff1a in the somites at 15 hpf. (B) Misexpression of mouse Shh RNA led to the expansion of the expression domain of ff1a. (C) ff1a expression is down-regulated in the trunk somites at 24 hpf, (D) ff1a RNA is retained in Shh overexpressed embryos. (E) Weak ff1a expression could be seen in control embryo at 20 hpf, (F) whereas in forskolin-treated embryos, ff1a expression is abolished in the somites. (G–J) Cross-sections of the embryos in panels (A, B) are shown in panels (G, H), respectively. Expression domain of ff1a in the somites at 18 hpf (I) was expanded in embryos where PKI was overexpressed (J). EXPRESSION / LABELING:
|
|
ff1a functions in slow myofibril assembly. Zebrafish embryos were injected with (A) β-gal (B, H) a deleted ff1a, dff1a (C, I), ff1a (D), control morpholino (MO) (E), ff1a morpholino or (F), ff1a morpholino plus ff1a mRNA, and then their slow myofibrils were stained with F59 antibody. (A–F) Lateral view of slow muscle fibrils in somites 14–17. The figures below them are magnified muscle fibrils. (A) Normal striated fibrils at 24 hpf. (B) Expression of dff1a results in the formation of thinner muscle fibrils. (C) Overexpression of ff1a leads to thicker fibrils with expanded width. (D) Control morpholino-injected embryo does not affect slow fibrils. (E) Embryos injected with ff1aMO have thinner fibrils. (F) Co-injection ff1a mRNA and ff1aMO rescued the phenotype caused by ff1aMO. (G–I) Electron micrographs of the third dorsal slow muscle fiber counting from the pioneer cells of the anal somite. (G) In the control fiber, myosin and actin filaments are organized into sarcomeres with the appearance of arrays of thick and thin black dots inside the sarcomeres (enlarged in the inserted red boxes). (H) In dff1a-injected embryo, myosin thick filaments and actin thin filaments are not well formed (inside red box), a few sarcomeres are not complete (red arrow), and in few sarcomeres, the myofibrils are not formed (green box). (I) Myosin rods are denser in ff1a overexpressed embryos (enlarged in the insert). |
|
Effect of ff1a on fast muscles. (A–C, G–I) Lateral view with anterior towards the left. (A–C) Expressions of Engrailed at 24 hpf (detected by 4D9 antibody, white arrows), (D–I) fast muscle Myosin Heavy Chain expression (MyHC, detected by zm4 antibody in red). (D–F) Cross-sections of the trunk. Slow muscle is detected by S58 antibody (green). Blue color indicates Hoechst staining for nuclei. (A, D, G) β-gal-injected embryos, (B, E, H) dff1a-injected embryos, (C, F, I) ff1a-injected embryos. Scale bars are 20 μm. The intensities of staining for both slow and fast muscles are reduced in dff1a-injected embryos and increased in ff1a-overexpressed embryos. |
|
Partial restoration of slow muscle myofibril organization in syu mutants by transient expression of ff1a. Slow myofibrils were detected by antibody F59. (A, B, E, F) Lateral view of somites 14–17, (C, D, G, H) cross-sections. The blue color is Hoechst dye staining for nuclei. (A) Normal slow muscle fibrils in the β-gal-injected wild-type (WT) embryo at 24 hpf. (B) Overexpression ff1a led to thicker slow myofibrils. (E) Distorted appearance of slow myofibrils in the syu mutant. (F) In the ff1a overexpressed syu mutant, the slow myofibrils are better organized and the width of the myofibril expanded. Scale bars are 20 μm. |
|
Partial restoration of muscle myofibril in ubo mutants by ectopic expression of ff1a. The ubo mutants were injected with (A, C) control β-gal or (B, D) ff1a RNA followed by staining with (A, B) S58 or (C, D) F59 antibodies at 24 hpf, and somites 14–17 are shown in lateral view with anterior towards the left. (A, B) Slow muscle specific staining of S58 confirmed that the differentiation of slow myofiber was partially restored by transient expression of ff1a in ubo mutants. (C) In the ubo mutants, very few myofibrils can be stained by F59. (D) Ectopic expression of ff1a resulted in the formation of more myofibrils at the surface. Arrowheads point to mono-nucleated myofibrils as shown in the enlarged figure below, including nuclear staining by DAPI. The arrows point to fast multi-nucleated myofibrils in the enlarged picture. The F59 antibody detects slow myofibrils strongly and fast myofibrils weakly. Scale bars are 20 μm. |
|
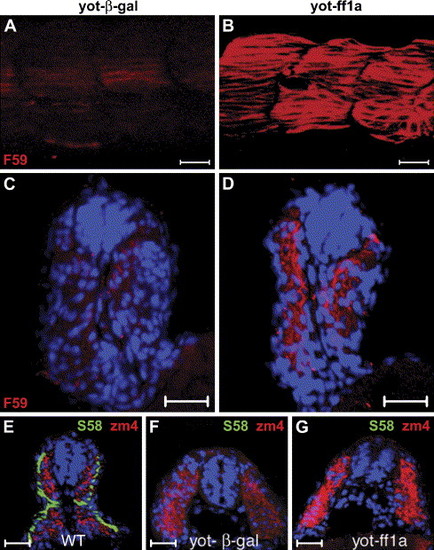
Injection of ff1a into yot mutants results in increased fast muscle myofibrils. The yot mutants were injected with (A, C, F) control β-gal or (B, D, G) ff1a RNA followed by staining with (A–D) F59 or (F, G) S58 plus zm4 antibodies at 24 hpf, and somites 14–17 are shown in lateral view with anterior towards the left. (A) The yot mutant reacted very weakly with F59 antibody at 24 hpf. (B) Ectopic expression of ff1a in yot mutants results in increased F59 staining. (C–G) Cross-sections. (C, D) The location of F59 staining (red) in the myotome indicates that these are fast muscles. Nuclei are stained with Hoechst dye (blue). (E) Slow muscle cells of normal siblings (WT) stained with S58 are arranged as a monolayer at the periphery of the somite, and fast muscle cells stained by zm4 are located more medially at 24 hpf. (F) There is no S58 staining and weak zm4 staining in control RNA-injected yot embryos. (G) Misexpression of ff1a causes an increase of zm4 staining in yot mutants, but S58 staining remains absent. Scale bars are 20 μm. |
|
Epistatic relationship of ff1a and prox1. Wild-type (WT) embryos were injected with control MO (A, E), prox1 MO (B, D, F), β-gal RNA (G, K), dff1a RNA (H, J, L), full-length ff1a RNA (C, D), or prox1 RNA (I, J). Gene expression was detected by in situ hybridization with ff1a probe (A, B), F59 antibody (red, A–D, G–J), or Prox1 antibody (green, A–D, K, L). (B) In Prox1 morphant, slow twitch myofibril is thinner. (C) Ectopic expression of ff1a did not perturb Prox1 expression, but the myofibrils as stained by F59 were expanded. (D) Co-injection of ff1a mRNA with prox1 MO restored the striation in myofibrils, although Prox1 staining is absent. Expression of ff1a in the slow muscle in the control (E) and the prox1 morphant (F) is not perturbed. (H) Knockdown of ff1a by dff1a RNA resulted in thinner myofibril. (K) Ectopic expression of prox1 mRNA in wild type embryos expanded the width of the myofibril. (J) When prox1 RNA was co-injected with dff1a RNA, slow myofibrils were thicker than those in panel (H) but thinner than those in panel (I). Expression of Prox1 is detected in (K) β-gal or (L) dff1a RNA-injected embryos. |

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 286(2), Sheela, S.G., Lee, W.C., Lin, W.W., and Chung, B.C., Zebrafish ftz-f1a (nuclear receptor 5a2) functions in skeletal muscle organization, 377-390, Copyright (2005) with permission from Elsevier. Full text @ Dev. Biol.