- Title
-
Zebrafish R-cadherin (Cdh4) controls visual system development and differentiation
- Authors
- Babb, S.G., Kotradi, S.M., Shah, B., Chiappini-Williamson, C., Bell, L.N., Schmeiser, G., Chen, E., Liu, Q., and Marrs, J.A.
- Source
- Full text @ Dev. Dyn.
|
Cdh4 knockdown affects eye size, body length, and brain morphology. A-H: Zebrafish embryos were injected (1-2 nl at 0.5 mM) with either standard control morpholino oligonucleotide (MO, A,B) or cdh4 MO (C-H). These injections produced a hypomorphic series of effects. At 2 days postfertilization (dpf), living embryos were imaged using a stereo-dissecting microscope (A,C,E,G) or by differential interference contrast microscopy (B,D,F,H). A-H: The cdh4 morphants (C-H) were micro-ophthalmic, had small lenses, lacked retinal lamination, had perturbed brain organization and shorter body length compared with controls (A,B). D,F,H: The fourth ventricle (IV) was enlarged and the hindbrain (hb) was reduced in cdh4 morphants. PHENOTYPE:
|
|
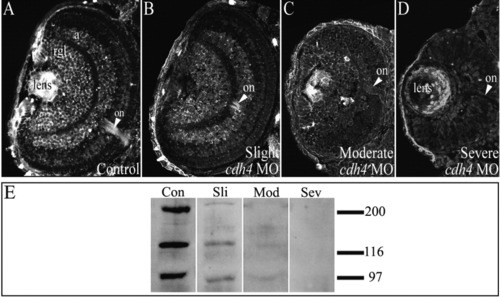
Eyes of cdh4 morphants are small and lack discrete laminae at 2 days postfertilization (dpf). A-K: Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, A,E,H) or cdh4 MO (B-D,F,G,I-K). A-D: At 2 days postfertilization (dpf), living embryos were imaged using differential interference contrast microscopy. Ventral views of eyes are shown (A-D). A: Rostral is up and lateral is left. In eyes of control embryos, all the major layers characteristic of the adult retina, the retinal ganglion cell layer (rgl), inner plexiform layer (ipl), the inner nuclear layer (inl), outer plexiform layer (opl) and the outer nuclear layer (onl) are visible. B-D: In cdh4 MO-injected embryos, a graded series of effects on eye development were seen. B,C: In moderately affected embryos, eyes were smaller in size, the inner plexiform layer was sparse, the distance between the inner plexiform layer and the lens was not uniform, and the outer nuclear layer was sparse or absent. D: Both the eyes and lens of severely affected embryos were small and laminae were altogether absent. E-G: BODIPY-ceramide labeled living 2 dpf embryos were imaged using confocal microscopy. E: In control embryos at 2 dpf, there was a distinct continuous inner plexiform layer separating the retinal ganglion cell layer and the inner nuclear layer. F: In moderately affected 2 dpf cdh4 MO knockdowns, the inner plexiform layer was sparse and irregular and the outer nuclear layer was absent. G: In severely affected 2 dpf cdh4 MO knockdowns, lamination was absent. H-K: Toluidine blue-stained plastic sections of 2 dpf embryos clearly show the laminar organization of cells within the control retina (H). I: In slightly affected cdh4 knockdowns, laminae are present, but cell shape, particularly within the outer nuclear layer differs from that of controls. J,K: In severe cdh4 knockdowns, laminae are absent. Scale bars = 50μm. PHENOTYPE:
|
|
Reduction of Cdh4 protein levels correlates with phenotypic severity of cdh4 morphants. A-D: Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, A) or cdh4 MO (B-D). A: Confocal imaging of 3 days postfertilization (dpf) Cdh4 immunostained cryosections showed Cdh4 localization in the retinal ganglion cell layer, in the amacrine cell layer of the inner nuclear layer, in the lens, and in the optic nerve of the control embryo. B,C: In slightly and moderately affected cdh4 MO-injected embryos, staining of the retinal ganglion cell layer, the amacrine cell layer and the optic nerve was reduced. D: In severely affected embryos, Cdh4 staining was not detected in the neural retina and reduced in the lens (the apparent immunoreactivity of the ectoderm and lens is nonspecific, that is, it could not be competed with excess immunizing peptide; Liu et al.,[2001b]). E: Corresponding immunoblots of individual embryos of various phenotypic severities in the hypomorphic series showed that, compared with control embryos at 3 dpf (con), Cdh4 expression was reduced in slightly and moderately affected embryos (sli, mod) and absent in severely affected embryos (sev). EXPRESSION / LABELING:
PHENOTYPE:
|
|
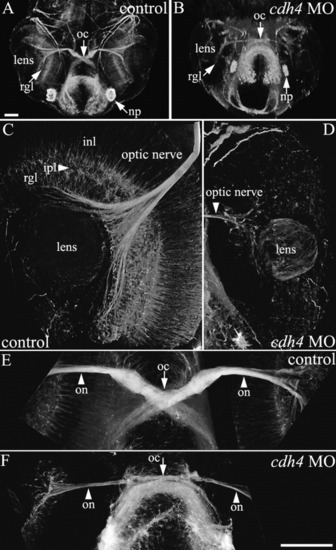
cdh4 knockdown results in sparse retinal ganglion cell axon outgrowth at 2 days postfertilization (dpf). A-F: Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, A,C,E) or cdh4 MO (B,D,F). Embryos were fixed at 2 dpf, and whole-mount preparations with axon tracts immunostained using acetylated tubulin antibodies were visualized using two-photon imaging. Ventral view projections are shown of the planes containing the eyes and optic nerves. A,B: Low-magnification views are shown for orientation. A: In control embryos, axons exit the retinal ganglion cell layer to form the optic nerve, which then bends to travel more dorsally and rostrally, crosses the midline at the optic chiasm, then projects straight toward the contralateral optic tectum. B: In severe cdh4 knockdowns, both the retinal ganglion cell layer and optic nerve are sparse and the bend in the optic nerve as it travels toward the optic chiasm is absent, but the sparse optic nerve does project normally across the midline toward the contralateral optic tectum. C,D: In high-resolution views of the control eye, extensive neural processes of all the major retinal layers are evident (C), whereas in the cdh4 knockdown eye, neuronal processes are largely absent and only a sparse disorganized retinal ganglion cell layer is visible (D). Projection images of two-photon image volumes are shown. E,F: High-resolution views of the optic chiasm show the thick well fasciculated optic nerve of the control (E) and the thin loosely fasciculated optic nerve of the cdh4 knockdown (F). Scale bars = 50 μm. PHENOTYPE:
|
|
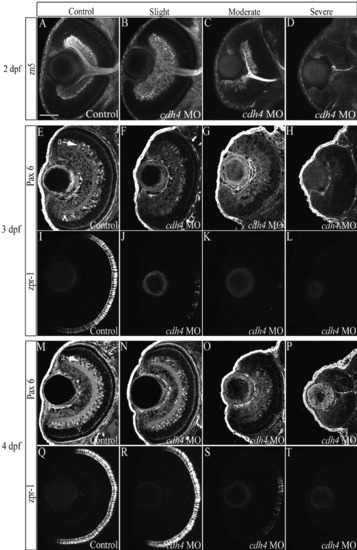
cdh4 knockdown affects differentiation of the retina. A-T: Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, A,E,I,M,Q) or cdh4 MO (B-D,F-H,J-L,N-P,R-T). Retinal ganglion cells were visualized at 2 days postfertilization (dpf), using confocal imaging of zn5-immunostained whole-mount embryos. Images are ventral view projections at the level of the optic nerve. Zn5 identifies retinal ganglion cells and their axon processes, among other cells in the developing zebrafish. A: In control embryos, the retinal ganglion cell layer was uniform in thickness and had smooth boundaries. B: The retinal ganglion cell layer was often expanded in cdh4 MO-injected embryos that were only slightly affected. C: In moderately affected embryos, the retinal ganglion cell layer was sparse with irregular boundaries and the optic nerve was thin. D: In severely affected cdh4 knockdowns (D), the retinal ganglion cell layer ranged from nearly absent (D), with only one or two zn5-positive cells and their projections, to altogether absent (not shown). E-T: For Pax6 and zpr-1 immunolocalization, embryos were fixed at 3 dpf (E-L) or at 4 dpf (M-T) and 8 μm horizontal cryosections were cut. Adjacent sections of the same eye (except H, L) were immunostained for Pax6-positive amacrine cells (E-H,M-P) and zpr-1-positive double cone photoreceptors (I-L,Q-T) and visualized by confocal microscopy. cdh4 knockdown images are arranged from left to right as a graded series of slightly affected (F,J,N,R), moderately affected (G,K,O,S), then severely affected (H,L,P,T) embryos. F,H,J,L,N-P,R-T: Amacrine cells and photoreceptor cells fail to differentiate in severe knockdowns (H,L,P,T), but, when present in less affected knockdowns (F,J,N,O,R,S), they were localized in appropriate retinal laminae. Scale bar = 50 μm. |
|
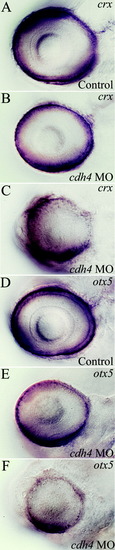
Expression of early markers of photoreceptor identity is reduced in cdh4 morphants. A-F: Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, A,D) or cdh4 MO (B,C,E,F). crx (A-C) and otx5 (D-F) labeling in 78 hours postfertilization (hpf), embryos were used as markers of photoreceptor specification and differentiation. A,D: In controls, crx and otx5 labeling were confined to the photoreceptor layer and the outermost part of the inner nuclear layer. B,E: Some reduction of staining was seen in slightly affected cdh4 morphants. C,F: In severe cdh4 morphants, both crx and otx5 staining were reduced and lacked defined laminae. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Microphthalmia is due to increased cell death in cdh4 knockdowns. A-D,F-M: Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, A,F,H,J,L) or cdh4 MO (B-D,G,K,I,M). Mitotic cells were visualized in eyes of 48 hours postfertilization (hpf) embryos using histone-H3 immunostaining (control, n = 31; slight, n = 16; moderate, n = 15; severe n = 13). A-D: In both control (A) and in cdh4 knockdowns (B-D), dividing cells were confined to the peripheral lamina of the retina. E: Comparison of the number of H3-positive nuclei per μm2 showed no significant difference between controls and cdh4 knockdowns. Statistical comparisons of these data were made in two ways. A two-tailed unpaired Students t-test was used to compare controls and pooled data from all cdh4 morphant groups (P = 0.07). One-way analysis of variance was used to compare controls and unpooled cdh4 morphant groups (P = 0.08). E: Chart shows unpooled group data. Error bars = 1 SD. A-D: Representative confocal images are shown of control (A) and cdh4 knockdowns from the slightly affected (B), moderately affected (C), and severely affected (D) cdh4 morphant groups. Dying cells were visualized using acridine orange (AO) vital staining at 36 hpf (control, n = 9; cdh4 MO, n = 14) and at 50 hpf (control, n = 5; cdh4 MO, n = 6). F-M: Representative differential interference contrast images (F-I) and a corresponding fluorescent image (J-M) are shown for each group; control at 36 hpf (F,J), cdh4 MO injected at 36 hpf (G,K), control at 50 hpf (H,L), and cdh4 MO at 50 hpf (I,M). AO-labeled cells were counted. Cell death was increased in the retina of cdh4 knockdowns compared with controls at both 36 hpf (P < 0.01) and 50 hpf (P < 0.01). Randomly selected embryos were used for AO staining to minimize potential bias. Cell death was increased in the lens at 50 hpf (P < 0.05) but not at 36 hpf. Error bars = 1 SD. |
|
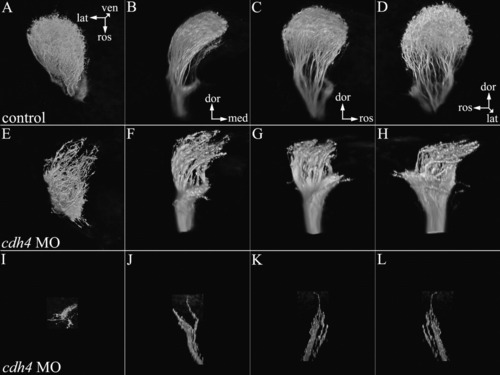
Retinal ganglion cell axons in 5 days postfertilization (dpf) cdh4 knockdowns project to the contralateral optic tectum but do not arborize normally. A-L: Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, A,D,G,J) or cdh4 MO (B,C,E,F,H,I,K,L). Embryos were fixed at 5 dpf and retinotectal projections were traced by injecting one eye in each embryo with the lipophilic dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate). Projection images of two-photon volumes of the DiI-filled retinotectal projections (shown in red) were overlaid with differential interference contrast (DIC) images from the same embryo (A-C). Staining within the injected eye was digitally removed to leave the DIC image of the eye visible. A-C: In controls (A), retinal ganglion cells project contralaterally and arborize to fill the optic tectum. In cdh4 knockdowns (B,C), retinal ganglion cells project to the contralateral optic tectum but do not arborize normally. D-L: These same three fish are shown at higher resolution. A-C: The DiI-injected fish were counterstained with DAPI (4′,6-diamidine-2-phenylidole-dihydrochloride) so that the boundaries of the tectal neuropil could be seen in relationship to the retinotectal projections (shown in gold). DAPI (shown blue-violet) stains the cell nuclei. Cellular regions are filled with nuclei, but nuclei are absent from regions of neuropil such as the optic tectum (ot). D,G,J: In controls (D,G,J) from a dorsal view (D,G), the retinotectal projections arborized to fill the entire optic tectum. J: A medial view of a sagittal cut through the optic tectum of the control volume shows the deep tectal layers. E,F,H,I,K,L: In cdh4 knockdowns, both retinotectal projections and the optic tectum were affected. E,H: Optic tecta in some moderately affected cdh4 knockdowns were apparently normal in size, but the retinotectal projections were sparse, disorganized, and did not fill the tectal neuropil. K: A medial view of a sagittal cut through the optic tectum of the moderately affected cdh4 knockdown lacks the well defined deep layers. F,I,L: In severe knockdowns, the tectal neuropil was smaller (F,I) and some neurites extended beyond the neuropil boundary and into the cellular layer (arrowheads I,L). Scale bars = 50 μm. PHENOTYPE:
|
|
Retinal ganglion cells in 3 days postfertilization (dpf) cdh4 knockdowns arborize only the lateral optic tectum or terminate before reaching the tectum. A-L: Zebrafish embryos were injected with either standard control morpholino oligonucleotide (MO, A-D) or cdh4 MO (E-L). Embryos were fixed at 3 dpf, and retinotectal projections were traced by injecting eyes with the lipophilic dye DiI (1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate). In both controls and cdh4 knockdowns, retinal ganglion cell axons project toward the contralateral optic tectum. In controls, upon reaching the optic tectum, retinal ganglion cells projections arborized extensively within the tectal neuropil. I-L: In severely affected cdh4 knockdowns, the optic nerve was sparse and failed to reach the optic tectum. E-H: In moderate cdh4 knockdowns, the optic nerve reached the optic tectum, but arborized only the more lateral region. In knockdowns, the nerve fibers were blunt and the fine branching seen in controls was absent. Multiple renderings are shown of each two-photon volume. A,E,I: Dorsal views of the optic tectum, with rostral down and lateral to the left. B,F,J: In the next view, the volumes have been rotated so that we are looking at a rostral view of the fish with dorsal up and lateral to the left. C,D,G,H,K,L: The upright volume renderings of B,F,J have been rotated counterclockwise. PHENOTYPE:
|
|
Retinal ganglion cells (RGCs) expressing Cdh4ΔC at 24-26 hours postfertilization (hpf) have few or no processes at 56 hpf. A,B,D,F,H: Photomicrographs from retinal sections. C,E,G: Photomicrographs from whole-mount embryos. A,B: Shown are Cdh4ΔC-expressing cells in the retina (A) and in trunk skeletal muscle fibers (B) that were heat shocked at 25-26 hpf. A: A cross-section of the eye (dorsal to the right and lateral above) showing Cdh4ΔC-expressing cells throughout the thickness of the retina. B: A cross-section (dorsal above and lateral to the right) of the body trunk region showing that the anti-myc immunostaining was located mainly in the periphery of each muscle fibers (mf). C,D,F: Control RGCs expressing either MT/eGFP (C,D) or eGFP (F). These RGCs have numerous dendrites (arrow) and each has an axon (arrowhead). A,G,H: RGCs expressing the dominant-negative Cdh4ΔC (heat shocked at 25-26 hpf) have many fewer or no processes. E: A Cdh4ΔC-expressing RGC (heat shocked at 48 hpf) with normal RGC morphology. gcl, retinal ganglion cell layer; hHS, hours heat shock; inl, inner nuclear layer; onl, outer nuclear layer; sc, spinal cord; eGFP, enhanced green fluorescent protein. |