- Title
-
The you Gene Encodes an EGF-CUB Protein Essential for Hedgehog Signaling in Zebrafish
- Authors
- Woods, I.G., and Talbot, W.S.
- Source
- Full text @ PLoS Biol.
|
you Mutants Exhibit Hedgehog-Associated Defects in Slow Muscle and Ventral Spinal Cord. (A and B) Lateral views of live zebrafish at 22 hpf. Wild-type embryos (A) have chevron-shaped somites and a clearly visible floor plate (arrowhead), while you mutants (B) exhibit U-shaped somites and an indistinct floor plate (arrowhead). (C and D) Lateral views of somites 8–13 in whole-mount embryos at 24 hpf. Wild-type embryos (C) show strong Engrailed expression in muscle pioneers (arrow), and weaker expression in multinucleate medial fast fibers (arrowheads). Engrailed expression in you mutants (D) is mostly absent, though very weak expression can occasionally be observed (arrowhead). (E and F) Dorsal views of posterior trunk and tail bud in 12-somite embryos. Wild-type embryos (E) exhibit adaxial myod expression throughout the somitic (arrowhead) and presomitic (arrow) mesoderm, while you mutants (F) lack expression in the somitic (arrowhead) and in parts of the presomitic (arrow) mesoderm. (G and H) Lateral view of somites 9–15 in whole-mount embryos at 22 hpf. Wild-type embryos (G) exhibit strong expression of ptc1, while you mutants (H) show weaker levels of ptc1 expression. (I and J) Lateral view of spinal cord in the posterior trunk of whole-mount embryos at 24 hpf. Wild-type embryos (I) show expression of nkx2.2 in the ventral spinal cord, while in you mutants (J) this expression is strongly reduced. (K–N) Dorsal views of whole-mount embryos at bud stage (10 hpf). Expression in you mutant embryos of both ehh (L) and shh (N) is similar to that observed wild-type embryos (K and M). Anterior is to the left in all images. Genotypes of all embryos were determined by PCR after photography. PHENOTYPE:
|
|
MO-Induced Phenocopy of you Defects and Rescue of the you Phenotype with mRNA Injection. (A, B, E, and F) Dorsal view of the posterior trunk and tail bud of 12-somite embryos. (C, D, G, and H) Lateral views of somites 8–13 in whole-mount embryos at 24 hpf. Anterior is to the left in all images. Injection at the 1–4-cell stage of 420 pg of a MO targeting the translational start site of the you mRNA (ATG MO) resulted in decreased adaxial expression of myod in the somitic (arrowhead) and presomitic (arrow) mesoderm of wild-type embryos (B). Injection of an equivalent amount of a mismatch control (mismatch MO) did not produce these defects (A). Similarly, wild-type embryos injected with 420 pg of the mismatch MO (C) exhibited strong Engrailed expression in muscle pioneers (arrow) and weaker expression in medial fast fibers (arrowheads). In contrast, Engrailed expression was strongly reduced in wild-type embryos injected with 420 pg of the ATG MO (D), though very weak expression was still observed (arrowhead). Genotypically you mutant embryos (E) showed rescued expression of adaxial myod in somitic (arrowhead) and presomitic (arrow) mesoderm when injected with 50 pg of synthetic you mRNA at the 1–4-cell stage, while mutants injected with 50 pg of a frameshift mutant form of you mRNA (F) did not exhibit rescue of adaxial myod expression. At 24 hpf, genotypically you mutant embryos injected at the 1–4-cell stage with 50 pg of you mRNA (G) showed rescue of strong Engrailed expression in the muscle pioneers (arrow) and weaker expression in the medial fast fibers (arrowheads). Mutant embryos injected with 50 pg of the mutant mRNA (H) did not show rescued Engrailed expression, though very weak Engrailed expression (arrowhead) was observed in some cases. Engrailed expression at the MHB was normal in all analyzed embryos (data not shown). Genotypes of embryos shown in (E–H) were determined by PCR after photography. EXPRESSION / LABELING:
|
|
Expression of you Examined by In Situ Hybridization. (A and B) Maternal you transcripts were evident in cleavage-stage embryos (A) (16-cell; 1.5 hpf), and you mRNA was widely expressed into the gastrula period (B) (shield stage; 6 hpf). In addition, you mRNA was detectable by RT-PCR at 2 hpf, prior to the zebrafish midblastula transition. (C) Toward the end of gastrulation, you transcripts began to be restricted, so that at the bud stage (10 hpf), you expression was evident in the eye field (white arrowhead), in the developing midbrain and hindbrain (black arrowheads), and in posterior paraxial stripes (arrow). (D) During early somitogenesis (12 hpf), you expression was observed in the eye field (white arrowhead), in stripes in the midbrain and the MHB (black arrowheads), in a complex pattern in the hindbrain (white arrow), and in paraxial stripes along the developing trunk and tail bud (black arrow). (E and F) At 24 hpf, you transcripts were observed dorsal to the hypothalamus (black arrow), at the boundary between the telencephalon and the diencephalon (white arrow), in the ventral tectum (white arrowhead), in the region of the presumptive cerebellum (asterisk), and dorsally along the length of the spinal cord. Additional expression of you at this stage and later was observed in the ventral tail and at the urogenital opening (arrowheads; data not shown). (G and H) At 48 hpf, you transcripts were highly expressed in the cerebellum (black arrow), and were also present in the rhombic lip (white arrowhead), and continuing along the length of the anterior–posterior axis in the dorsal spinal cord (black arrowhead; data not shown). Orientation of images: (A) lateral view; (B) lateral view, dorsal to the right; (C, D, F, and G) dorsal views, anterior to the left; (E and H) lateral views, anterior to the left. |
|
Early Overexpression of you in Wild-type Embryos and Rescue of you Defects by shh mRNA Injection. (A–D) Dorsal views of the posterior trunk and tail bud of whole-mount embryos at 12 somites (15 hpf). (E–F) Lateral views of somites 2–7 in 24-hpf embryos. (G–H) Lateral views of somites 8–13 in 24-hpf embryos. (I–L) Lateral views of 24-hpf embryos. Anterior is to the left in all images. When 50 pg of you mRNA was injected into wild-type embryos at the 1–4-cell stage, no obvious expansion of myod (B), Engrailed (F), or nkx2.2 (J) expression was observed when compared either with wild-type embryos injected with equivalent amounts of mutant mRNA (A, E, and I) or with uninjected embryos (see Figure 1). Muscle pioneers were counted in a subset of the embryos; there were 4.0 ± 0.8 Engrailed-expressing muscle pioneers per somite in embryos injected with the control mRNA (n = 3 embryos, 33 somites) and 4.6 ± 1.1 muscle pioneers per somite in embryos injected with synthetic you mRNA (n = 8 embryos, 88 somites). Injection of 50 pg of shh mRNA into embryos at the 1–4-cell stage resulted in expansion of myod, Engrailed, and nkx2.2 expression in both wild-type (C, G, and K) and you mutant (D, H, and L) embryos. shh injection rescued adaxial expression of myod (D), muscle pioneer expression of Engrailed (H), and ventral spinal cord expression of nkx2.2 (L) in genotypically mutant you embryos (compare with Figure 1). Genotypes of all embryos were determined by PCR after photography. |
|
Knockdown of patched Function Rescues Slow Muscle Defects in you. After injection of 420 pg of a mismatch control ptc1 MO, adaxial expression of myod (A) and Engrailed (B) was normal in wild-type embryos, but absent in you mutant embryos (C and D). When injected with 420 pg of a MO targeting ptc1, however, myod expression in mutants (E) was rescued to levels comparable to wild-type embryos (G). Engrailed expression was slightly expanded in both wild-type (F) and mutant (H) embryos injected with 420 pg of ptc1 MOs. Both adaxial myod expression and Engrailed expression was slightly expanded in wild-type (I and J) and you mutant embryos (K and L) injected with MOs targeting both ptc1 and ptc2 (420 pg each). Embryos assayed for myod expression are shown in flat mount at the 12-somite stage, and somites 5–9 of Engrailed-expressing embryos are shown in lateral view at 24 hpf. Anterior is to the left in all panels. Genotypes of all embryos were determined by PCR after photography. EXPRESSION / LABELING:
PHENOTYPE:
|
|
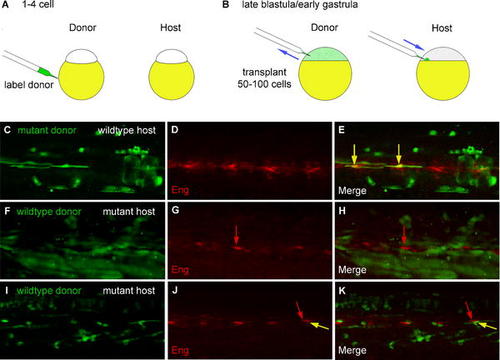
you Acts Non-Autonomously in Muscle Pioneers and Is Not Required in Cells Producing Hedgehog Signals. (A) Donor embryos were labeled at the 1–4-cell stage with Oregon Green dextran. (B) Cells from donor embryos were transplanted into unlabeled hosts during late blastula and early gastrula stages. (C–E) Images from a chimera made by transplanting cells from labeled mutant donors (green in C and E) into unlabeled wild-type hosts. At 24 hpf, muscle pioneer cells in chimeric embryos were labeled with anti-Engrailed antibody (red in D and E). When transplanted into wild-type embryos, mutant cells were able to differentiate as muscle pioneers, as shown by co-labeling with the anti-Engrailed antibody (E, yellow arrows). (F–K) Images from chimeras made by transplanting cells from labeled wild-type donors (green in F, H, I, and K) into unlabeled mutant hosts. Expression of Engrailed (red in G, H, J, and K) in some mutant muscle pioneers (one marked by red arrows in G, H, J, and K) was rescued in a subset of embryos (see also Table 1). Donor cells in the embryo shown in (F–H) contributed solely to muscle and to non-floor-plate identities within the neural tube. Moreover, in a subset of chimeras, cells derived from wild-type donors differentiated as muscle pioneer cells (yellow arrows in J and K), simultaneously showing both the characteristic strong nuclear Engrailed expression and the typical flattened and mononucleate morphology of this cell type. The somite labeled with the arrows in J and K contains two muscle pioneers, one derived from the wild-type donor (yellow arrow) and another derived from the mutant host (red arrow). Donor cells in the embryo shown in (I–K) contributed primarily to muscle and to non-floor-plate identities within the neural tube; in addition, a group of seven floor plate cells derived from the wild-type donor was present in the tail of this embryo (not shown). |