- Title
-
Formation of the retinotectal projection requires Esrom, an ortholog of PAM (protein associated with Myc)
- Authors
- D'Souza, J., Hendricks, M., Le Guyader, S., Subburaju, S., Grunewald, B., Scholich, K., and Jesuthasan, S.
- Source
- Full text @ Development
|
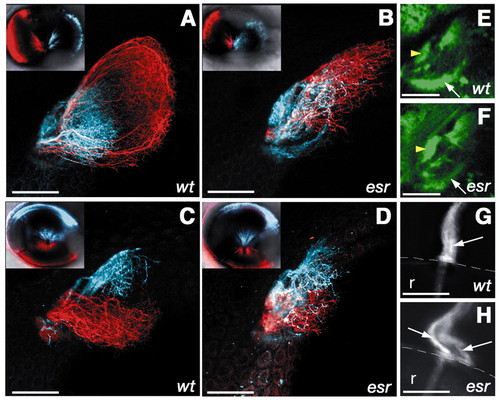
The retinotectal projection in the esrom mutant. (A-D) Optic tectum of 4-day-old zebrafish, with retinal axons labeled using DiI (red) or DiD (blue). Insets show lateral view of the injected eye. (A) Axons from the anterior region of the eye (red) project to the posterior tectum in wild type. Axons from the posterior (blue) arborize in the anterior tectum. (B) In the esrom mutant, anterior axons defasciculate and arborize anteriorly. (C) Axons from the ventral (red) and dorsal (blue) regions of the eye are well separated in a wild type. (D) In the mutant, there is some overlap between two populations. (E,F) Dorsal view of the pretectal region of 3-day-old fish, in which retinal axons are labeled with GFP under the sonic hedgehog promoter. The pretectal target AF7 (yellow arrowhead) is more innervated in the mutant (F) compared with the wild type (G). AF9 (arrow), by contrast, is less innervated in the mutant. (G,H) The optic nerve exiting from the eye in 7-day-old fish. Only dorsal neurons have been labeled with DiI. In the wild type (G), axons remain closely associated with one another in the optic nerve (arrow). In the mutant (H), axons have formed two separate bundles (arrows). Scale bar: 50 µm. Anterior is towards the left. r, retina; the broken white line in G,H indicates the margin of the eye. (E,F) Single optical planes; other panels are projections of z-stacks. |
|
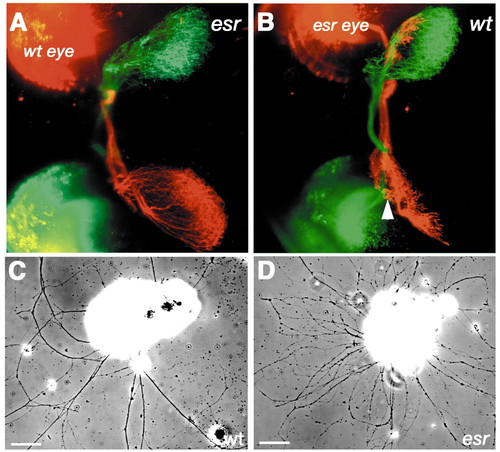
Autonomy of esrom gene function. (A) Transplantation of a wild-type eye into a mutant host. Axons from the anterior region of the wild-type eye (red) remain fasciculated and project to the posterior tectum. Axons from the host eye (green) branch aberrantly and are not tightly fasciculated in the optic tract. (B) Transplantation of a mutant eye into a wild-type host. Axons from the anterior region of the host eye (green) project to the posterior tectum, whereas those from the mutant eye (red) terminate in the anterior tectum. AF7 is indicated by the arrowhead. (C) Wild-type retinal axons fasciculate in vitro. (D) Mutant axons do not fasciculate as strongly. Scale bar: 50 µm. Anterior is towards the left. |
|
Positional cloning of esrom. (A) Map of zebrafish chromosome 9, depicting the region to which esrom maps and its synteny to human chromosome 13 (orange). Yellow color indicates synteny to human chromosome 2. (B) Genomic contig assembly of zebrafish PAC and BAC clones encompassing esrom and its similarity to human chromosome 13q22.3. esr maps 0.5 cM from z6663, 0.2 cM from fj33d03 and less than 0.08 cM from fa97c06. (C,D) Retinotectal projections of DiI-labeled anterior RGC axons in 4-day-old wild-type embryos injected with control morpholino and zebrafish PAM morpholino. (C) In the control, anterior RGC axons branch in the posterior tectum as in wild types. (D) In zebrafish, PAM morphants anterior RGC axons branch prematurely in the anterior tectum (arrow). (E,F) Lateral view of 3-day-old larvae. Xanthophore phenotype in morphants mimics that of mutants (Le Guyader et al., 2004). (G) Splice site target of zebrafish PAM morpholino2. (H) RT-PCR of 400 bp unspliced transcript in control and morphants. The unspliced band is amplified only in the zebrafish PAM morphants. (I) Single cDNA band (13 kb) from RT-PCR using esrom specific primers. (J) Point mutation in esrtp03. Scale bar: 25 µm. |
|
The esrom gene. (A) Predicted domains in Esrom. See text for full description. mo represents target sites for morpholinos. The positions of three mutations are indicated above the protein. (B) Esrom is phylogenetically closer to human PAM than Drosophila HIW and C. elegans RPM-1. (C) E3 ligase activity of 45 kDa Esrom C terminus containing the RING finger. Lanes 1-3 are controls. Lane 4 shows protein polyubiquitination in the presence of E1, E2 (UbcH5b) and Ub. (D,E) In situ hybridization with 30-hour-old zebrafish, shown here in lateral view. The antisense probe to esrom shows widespread label, with signal visible in all regions of the brain (D); no signal was detected with a sense probe (E). (F,G) Three-dimensional reconstructions of the eye of 48-hour-old fish labeled with antisense (F) or sense (G) probes to esrom, showing a lateral view. The gene is expressed uniformly in the developing retina. Lens labeling is nonspecific. (H,I) Western blot using a C terminus specific anti-PAM antibody. The antibody recognizes the 67 kDa C terminus end of Esrom (H), which is 98% identical to PAM, and a single 500 kDa protein from zebrafish embryo extract (I). (J-M) Esrom localization in retinal cells in vitro. (J,K) Label is detectable in the cytoplasm (arrowhead) and entire axon (arrow). Puncta are visible at high magnification (M, arrowhead). (J,L) Phase images; (K,M) the corresponding fluorescence images. (N-Q) Pre-absorption control; both axons were imaged at identical settings on a confocal microscope. (N,O) Without pre-absorption, the entire axon was labeled. (P,Q) With pre-absorption, very weak label was detected. Scale bar: 5 µm in L,M; 10 µm in J,K,N-Q; 100 µm in D-G; l, lens |
|
Branching in the retinal axons. Tectal arbors of RGCs expressing unc76-egfp in 4-day-old wild type (A) and mutant (B) are similar, with no measurable differences in branch number, arborization area or maturity index (D, error bars=s.e.m.). A subset of mutant axons stop near the tectal entry point at 3 days and do not arborize (C, arrow); the axon on the left was seen to arborize normally subsequently. Scale bar: 10 µm. |
|
In situ binding of EphrinB2-fc in the eye of a 4-day-old wild type (A) and esr te50 (B) embryo. Scale bar: 20 µm; eye is shown in lateral view, with anterior towards the left. |
|
Ser939 Phospho-Tuberin (green) and -tubulin (red) immunofluorescence in retinal explants. (A) After 15 hours in culture, Phospho-Tuberin (arrowheads) is detected in retinal axons, with higher levels in the esrom mutant (B). (C) At this time, Phospho-Tuberin immunofluorescence is twofold higher on average in esrom axon tips (*P<0.0001); box plot shows medians and quartiles from two normalized independent experiments (n wt=83, n esr=99 and n wt=26, n esr=78). After 26 hours in culture, the fasciculation phenotype is apparent, and Phospho-Tuberin staining difference is intensified. Scale bar: 50 µm |