- Title
-
Prep1.1 has essential genetic functions in hindbrain development and cranial neural crest cell differentiation
- Authors
- Deflorian, G., Tiso, N., Ferretti, E., Meyer, D., Blasi, F., Bortolussi, M., and Argenton, F.
- Source
- Full text @ Development
|
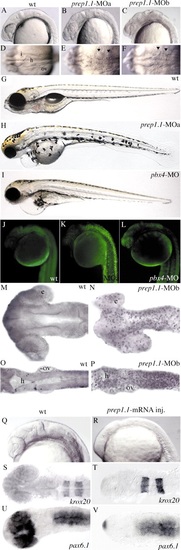
Expression of prep1.1 transcripts during development. (A) Dendrogram, obtained using the ClustalW program, of zebrafish Meis1.1, Meis2.1, Meis3.1 and Prep1.1, human PREP1 and PREP2 (hPREP1 and hPREP2), and Drosophila Homotorax (drHTH) protein sequences. (B) RT-PCR amplifies prep1.1 transcripts in zebrafish embryos at all stages from 2-16 cells to 4 dpf, and in ovaries of mature females. (C-I) RNA in situ hybridization of wild-type embryos. (C-F) prep1.1 transcripts are distributed ubiquitously up to 18 hpf. Arrows in E indicate head mesoderm. (G-I) From 24 hpf onwards prep1.1 transcripts concentrate in the head and become restricted to the region rostral to the otic vesicles (ov) at 72 hpf. Embryo in D is in lateral view with ventral to the left. Embryo in E is in frontal view. Embryos in F-H are in dorsal view and embryo in I is in lateral view. e, eye; m, midbrain; h, hindbrain; t, telencephalon. |
|
Subcellular localization of Prep1.1-GFP. (A) Full-length zebrafish prep1.1, as sequenced from the EST fc13f10, is represented with the Meis family homology regions (HR1 and HR2) in blue and the homeodomain homology region (HD) in red. The positions of the morpholinos (MOs) relative to the cDNA sequence are indicated by orange bars. prep1.1 constructs, with GFP coding sequence in green, are also shown. prep1.1-MOb inactivates endogenous, full-length mRNA but not the full-coding constructs. (B-J) Subcellular localization of Prep1-1-GFP chimeric proteins. (B-D,H) In a wild-type embryo injected with 10 pg of prep1.1-GFP mRNA, Prep1.1-GFP, which is cytoplasmic at the high stage (not shown), is translocated to the nucleus from the sphere stage onwards. Starting from the shield stage, the fluorescent protein is restricted mostly to the nucleus. (E-G) In a pbx4 morphant injected with 6 ng pbx4-MO and 10 pg prep1.1-GFP mRNA, Prep1.1-GFP is also translocated to the nucleus from the sphere stage onwards, but significant amounts are detected in the cytoplasm in all subsequent stages. (I,J) In wild-type embryos, Prep1.1ΔHD-GFP, a derivative lacking the homeodomain, is translocated normally to the nucleus (I), whereas Prep1.1ΔHR-GFP, a derivative lacking the Meis homology region is not (J). (B-D,H) and (E-G) are from the same wild-type and pbx4 morphant embryos, respectively. (K) Western blot of extracts from 24 hpf zebrafish embryos. The left panel is a control blot to assess the migration of two Pbx proteins, mPbx1a and mPbx1b, of known molecular weight and antibody specificity. The two proteins, which are recognized specifically by the pan-Pbx antibody, were translated in vitro with hPrep1 in a rabbit reticulocyte system. The middle panel is another control using nuclear extracts (NE) and cytoplasmic extracts (CE) of either human HEK293 cells or mouse testis. The right panel shows the immunoblotting analysis of NE and CE from wild-type zebrafish embryos at 24 hpf and following injection of prep1.1-MOb. The migration of Pbx2 and Pbx4 (arrows) is inferred on the basis of the Mr of the bands, and observation of the identical pattern elsewhere (Waskiewicz et al., 2002). A pan-Pbx antibody (Pöpperl et al., 2000) was used throughout. |
|
The phenotype of prep1.1 morphants is characterized by apoptosis. (A-I) 24-hpf and 5-dpf embryos. (B,C,E,F) Embryos injected with either prep1.1-MOa or prep1.1-MOb have similar phenotypes, characterized by alterations in brain morphology and widespread cell death, which is clearly visible as a higher opacity that is particularly evident at the level of the hindbrain (arrowheads in E,F). (H) prep1.1 morphants are characterized by small head and eyes, lack of jaws, abnormal distribution of melanocytes and delayed reabsorption of the yolk sac. (I) pbx4 morphants are characterized by small head and eyes and reduced jaws. Both prep1.1 and pbx4 morphants display a swollen pericardium and lack an inflated swim bladder. (A-C,G-I) are lateral, and (D-F) are dorsal views, with anterior to the left. (J-L) Acridine orange staining reveals widespread cell death in prep1.1 morphants, but not in wild-type embryos and pbx4 morphants. Embryos are in lateral views. (M-P) TUNEL labelling reveals intense DNA fragmentation in the brain of prep1.1 morphants at the 22-somite stage, which is more pronounced in the hindbrain. Embryos are in dorsal views with anterior to the left. (Q-V) Injection of prep1.1 mRNA posteriorizes the zebrafish embryo. Visual inspection (Q,R), and in situ hybridization with krox20 (S,T) and pax6.1 (U,V) antisense probes reveals that embryos injected with prep1.1 mRNA (R,T,V) have a reduced forebrain compared to uninjected embryos (Q,S,U). e, eye; h, hindbrain; I, isthmus; ov, otic vesicle. PHENOTYPE:
|
|
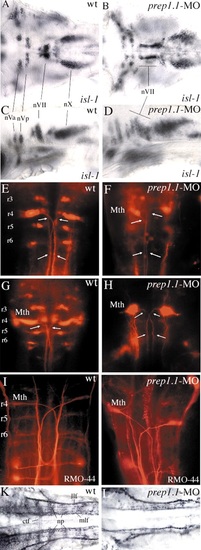
RNA in situ hybridization reveals disruption of hindbrain segmentation and patterning in prep1.1 morphants. (A,B) The pattern of hoxb1b expression is the same as wild type (wt) in prep1.1-MO-treated embryos. AP indicates the animal pole, V and D indicate the ventral and dorsal side of the embryo respectively. (C,D) In prep1.1 morphants, mariposa expression is abolished in the six rhombomere boundaries (from r1-r2 to r6-r7). (E,F) prep1.1 morphants lack the two rows of pax2.1-expressing commissural interneurons in the r2-r6 region of the hindbrain (indicated by asterisks). (G-I) The six rhombomere boundaries revealed by pax6.1 expression (from r1-r2 to r6-r7, indicated by asterisks in the wild-type embryo) are completely lost in prep1.1 morphants and only the r4-r5, r5-r6 and r6-r7 boundaries (asterisks) are clearly identifiable in pbx4 morphants. (J,K) hoxb1a expression in r4 is lost in prep1.1 morphants. (L-O) The levels of expression of hoxa2 (r2-r5) and hoxb2 (r3-r5) are severely reduced in prep1.1 morphants. (P-S) In prep1.1 morphants, krox20 expression is reduced only in r3; injection of prep1.1-MOb with a prep1.1 mRNA construct that lacks the sequence complementary to prep1.1-MOb rescues the wild-type phenotype. In pbx4 morphants, krox20 expression in r3 is also very weak. (T,U) Co-injection of pbx4/lzr mRNA in prep1.1 morphants partially rescues hoxb1a levels but does not rescue krox20 in r3. Embryos are stained at 60% epiboly (A,B), 20 hpf (J,K), 24 hpf (C-I,L-S). All embryos, except A and B, are in dorsal views with anterior to the left. Abbreviations: ov, otic vesicle; ncc, neural crest cells. The animals represented in this figure are different from those of Table 1. Quantitative data: (picture) probe, defective/total; (B) hoxb1b, 2/21; (D) mariposa, 21/26; (F) pax2.1, 25/30; (H) pax6.1, 16/18; (K) hoxb1a, 18/21; (M) hoxa2, 17/22; (O) hoxb2, 19/27; (Q) krox20, 19/25; (R) krox20, 7/20 (rescue P<0.01); (S) krox20, 19/23; (T) hoxb1a, 5/11 (rescue P<0.05); (U) krox20, 11/18. PHENOTYPE:
|
|
Motor nuclei and ganglia of cranial nerves and reticulospinal neurons, and neuronal architecture of the hindbrain in prep1.1 morphants. (A-D) At 48 hpf, isl1 expression is not affected quantitatively in the motor nuclei of the cranial nerves in prep1.1 morphants (B,D), but the motor neurons of the facial nerve (nVII) have not migrated caudally as in wild-type embryos (A,C). (E-H) Retrograde labelling with rhodamine-dextran of 3-day-old (E,F) and 5-day-old (G,H) embryos. In prep1.1 morphants, only r4 cells are labelled; the projection of their axons (arrows) is similar to that of Mauthner cells but their morphology is slightly different. (I,J) RMO-44 antibody staining of reticulospinal neurons at 48 hpf. Only Mauthner cells are present in prep1.1-MO injected embryos. (K,L) At 30 hpf, the staining pattern of acetylated tubulin antiserum shows that the neuropils and commissural tract fibres, (which are mostly out of focus in wild-type embryos) are much reduced or absent in prep1.1 morphants. Embryos are in dorsal (A,B,E-L) or lateral (C,D) views, with anterior to the left (A-D,K,L) or the top (E-L). ctf, commissural tract fibres; llf, lateral longitudinal fascicle; Mth, Mauthner cells; mlf, medial longitudinal fascicle; np, neuropil; nVa and nVp, anterior and posterior clusters of the trigeminal motor nucleus; nVII and nX, motor nuclei of the facial and vagus nerves, respectively; r, rhombomere; wt, wild-type. |
|
prep1.1 morphants lack the pharyngeal skeleton. Alcian blue staining of the head skeleton of 5 day-old prep1.1 morphants (C,D) reveals the complete lack of all pharyngeal cartilages, while those of the neurocranium are reduced. The wild-type phenotype can be substantially rescued by coinjection of prep1.1-MOb with a prep1.1 mRNA construct lacking the sequence complementary to MOb (E,F). In pbx4 morphants (G,H), the mandibular and hyoid cartilages are present, but are aberrant in shape and size and abnormally fused (arrowhead). Larvae are in lateral (A,C,E,G) and ventral (B,D,F,H), views with anterior to the left. cb, ceratobranchial; ch, ceratohyal; e, ethmoid plate; hs, hyosymplectic; me, Meckel′s cartilage; n, notochord; oa, occipital arch; pch, parachordal; pq, palatoquadrate; tr, trabeculae cranii. PHENOTYPE:
|
|
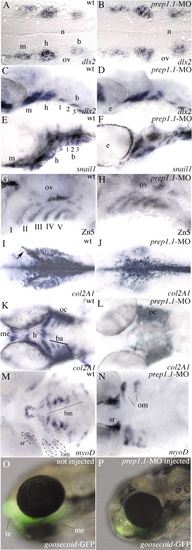
Neural crest cells migrate to the pharyngeal arches in prep1.1 morphants but do not differentiate into chondrocytes. (A,B) At 19 hpf (20-somite stage), dlx2 expression reveals three discrete clusters of neural crest cells migrating to the mandibular (m), hyoid (h) and branchial (b) arches in wild-type embryos and prep1.1 morphants. (C,D) At 33 hpf, in both wild-type and prep1.1-MO-injected embryos the dlx2-positive neural crest cells have reached the target position; the branchial cluster has split into three subgroups of cells. (E,F) At 48 hpf, snail1-expressing cells have segregated in the segmented pharyngeal arches both in wild-type embryos and prep1.1 morphants. (G,H) At 48 hpf the pharyngeal endoderm, revealed by the Zn5 antibody, is correctly formed in prep1.1 morphants. (I,J) At 28 hpf, col2a1 expression in prep1.1-MO injected embryos reveals normal differentiation of neurocranium cartilages. However, the mesenchyme originating the parachordalia of the neurocranium is reduced (cells indicated by an arrow in I). (K,L) At 48 hpf, in the pharyngeal region of prep1.1 morphants col2a1 is expressed only in the otic capsule; expression is lacking in branchial arches. (M,N) At 3 dpf, in the pharyngeal region of prep1.1 morphants myoD is expressed only in opercular muscles and superior rectus of the eye; expression is lacking in branchial muscles. (O,P) prep1.1 knockdown in gsc::GFP transgenic larvae suppresses GFP expression in the first pharyngeal arch but not in the anterior telencephalic area. Embryos are in dorsal (A,B,I-N) and lateral (C-H,O,P) views, with anterior to the left. b, branchial arch; ba, branchial arches; bm, branchial muscles; h, hyoid arch; m, mandibular arch; me, Meckel′s cartilage; n, notochord oc, otic capsule; om, opercular muscles; ov, otic vescicle; sr, superior rectum of the eye; te, telencephalon. Quantitative data: (picture) probe, defective/total; (B) dlx2, 1/18; (D) dlx2, 0/22; (F) snail1, 4/25; (H) zn5, 4/18; (J) col2a1, 18/21; (L) col2a1, 18/19; (N) myoD, 16/18; (P) gsc, 4/5. EXPRESSION / LABELING:
|

Unillustrated author statements |