- Title
-
Essential role for puma in development of postembryonic neural crest-derived cell lineages in zebrafish
- Authors
- Parichy, D.M., Turner, J.M., and Parker, N.B.
- Source
- Full text @ Dev. Biol.
|
Wild-type and puma mutant adult zebrafish. (A) Wild-type fish develop a series of alternating light and dark horizontal stripes. Dark stripes include melanophores, iridophores, and occasional xanthophores (Parichy and Turner, 2003b). Light stripes include xanthophores, iridophores, and occasional melanophores. Primary adult melanophores stripes develop during pigment pattern metamorphosis immediately dorsal (1D) and ventral (1V) to a lighter interstripe region. During terminal stages of metamorphosis, dorsal melanophores population develop scales (S) and contribute a dark cast to the dorsum of the fish. At the end of pigment pattern metamorphosis, additional secondary adult melanophores stripes start to form, initially ventral to the first ventral primary melanophore stripe (2V1), and then both dorsally (2D) and ventrally (2V2), until on average five stripes are present. (B) In puma mutants, melanophore numbers are severely reduced during early and middle stages of pigment pattern metamorphosis, and adult melanophores typically only appear during stages corresponding to the terminal phase of pigment pattern metamorphosis in wild-type fish. Normal primary stripes do not form, but as new melanophores appear late in development and cover dorsal scales (S), a striped pattern is partially recovered, yielding a pattern of regulatory melanophore stripes (R) that varies in completeness. Scale bar, 2 mm. |
|
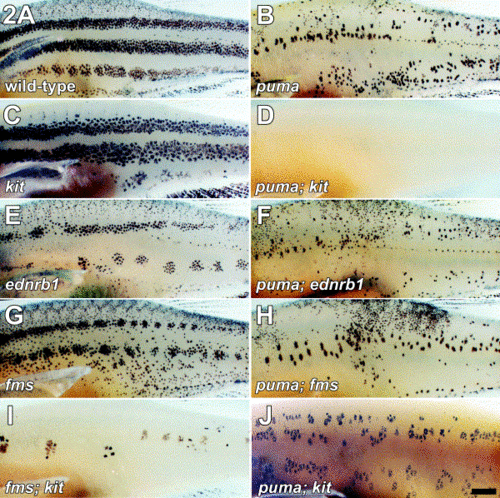
Epistasis analyses reveal an essential role for puma in promoting early- and late-appearing metamorphic melanophore populations. (A) Wild-type adults exhibit a pattern of several horizontal stripes. (B) puma mutant adults exhibit patchy distributions of melanophores and the stripe pattern is not well-formed. (C) kit mutants lack early-appearing metamorphic melanophores and retain late-appearing melanophores that differentiate already in the position of adult stripes. kit mutants also lack most dorsal scale melanophores (Johnson et al 1995 and Parichy et al 1999). (D) puma; kit double mutant lacks virtually all melanophores, indicating a role for puma in the development of residual late-appearing melanophores normally present in the kit mutant. (E) ednrb1 mutants lack the late-appearing metamorphic melanophore population and retain instead early-appearing metamorphic melanophores. (F) puma; ednrb1 double mutant exhibits a more severe melanophore deficit than ednrb1 single mutant, suggesting that puma is essential for the normal development of residual early-appearing melanophores normally present in ednrb1 mutants. (G) fms mutant also lack some late-appearing metamorphic melanophores and retains early-appearing metamorphic melanophores. (H) puma; fms double mutant provides an independent test of whether puma promotes the development of early- appearing metamorphic melanophores. Similar to puma; ednrb1 double mutants, puma; fms double mutants have significantly fewer melanophores than fms single mutants, confirming a role for puma in the development of the early-appearing melanophore population. (I) fms; kit double mutant exhibits a gross reduction in melanophore numbers compared to either single mutant. (J) puma; kit double mutant at three months shows that many melanophores are regained during juvenile and adult development, despite the nearly complete absence of these cells immediately after metamorphosis. Images are of fixed specimens. Scale bars, (A–I) 1 mm, (J) 1 mm. |
|
puma mutant phenotype is temperature-dependent. (A) puma mutant reared at 24°C. (B) puma mutant reared at 33°C; a more severe melanophore deficiency is evident in comparison with (A). Scale bar, 500 μm. |
|
puma promotes xanthophore development. (A–C) Consecutive images from the flank of wild-type metamorphosing larva, showing progressive increase in xanthophore density within the developing interstripe region. (D–F) Consecutive images from the flank of a puma mutant larva showing that xanthophore densities increase with time, but are reduced compared with wild-type larvae at corresponding stages. In contrast to metamorphosis, xanthophore deficiencies are not apparent in puma mutant embryos. (A, D) 20 dpf; (B, E) 26 dpf; (C, F) 35 dpf. Scale bar, 100 μm. |
|
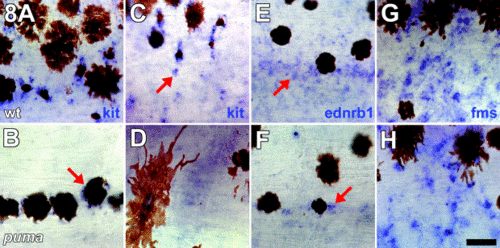
puma promotes normal numbers of cells expressing markers of pigment cell lineages. All images are side views of whole-mount larvae during middle stages of pigment pattern metamorphosis with anterior to the left. (A, C, E, G) Wild-type larvae. (B, D, F, H) puma mutant larvae. (A–D) puma mutants exhibit fewer kit+ presumptive melanoblasts and melanophores as compared with wild-type. (A, C) In wild-type larvae, staining for kit transcripts is evident in cell peripheries, and unmelanized kit+ presumptive melanoblasts are scattered over the flank (C, arrow). (B, D) In puma mutant larvae, kit staining is detectable in melanophores (B, arrow), but unmelanized kit+ are dramatically reduced in number. (E, F) puma mutants exhibit fewer ednrb1+ pigment cell precursors. (E) In wild-type larvae, ednrb1+ cells are widely scattered over the flank, and these cells may represent precursors to melanophores, xanthophores, or iridophores; ednrb1 staining is typically patchy and cell boundaries are not easily detectable (arrow; Parichy et al., 2000b). (F) In puma mutants, fewer ednrb1+ cells are detectable during early stages of pigment pattern metamorphosis (arrow), though these cells are recovered during later stages concomitant with the recovery of xanthophores. (G, H) puma mutants exhibit marginally fewer fms+ presumptive xanthophore precursors. (G) In wild-type larvae, numerous fms+ cells are present both in developing interstripe regions and, to a lesser extent, within developing melanophore stripes (also see Parichy and Turner, 2003b). (H) puma mutants exhibit a mild deficit of fms+ cells, consistent with the reduced xanthophore population, as compared with wild-type larvae. By later stages of pigment pattern metamorphosis, puma mutants do not exhibit detectably fewer fms+ cells compared with wild-type larvae (data not shown). Scale bar, 40 μm. |
|
puma promotes development of sox10-expressing cells but is not essential for normal foxd3 expression during metamorphosis. All images are side views of whole-mount larvae during middle stages of pigment pattern metamorphosis with anterior to the left. (A–H) mRNA in situ hybridization for sox10. (I–L) In situ hybridization for foxd3. (A, C, E, G, I, K) Wild-type larvae. (B, D, F, H, J, L) puma mutant larvae. (A) In wild-type larvae, sox10+ presumptive glia are abundant around the posterior trunk lateral line nerve (arrow). Melanophores can be seen in the skin, above the plane of focus. (B) In puma mutants, a severe reduction in sox10+ cells is evident particularly along the posterior trunk lateral line nerve. Only a few sox10+ cell are present at this location initially (arrow), though this number increases during later development. (C, E) Lower magnification views showing the flank of a wild-type larva, either superficially just beneath the skin (C), or deeper within the myotomes (E). Scattered sox10+ cells are evident in both locations, as well as lining the posterior trunk lateral line nerve (near the tops of the panels). (D, F) Corresponding views of an equivalent stage puma mutant larva showing the near complete absence of sox10+ cells both superficially and internally. A single sox10+ within the myotome is indicated (F, arrow). (G, H) Deeper views, just lateral to the larval midline. (G) In wild-type larvae, sox10+ presumptive glia are present in dorsal root ganglia (arrow), adjacent to the neural tube (nt). v, vertebrae. A few melanophores are present dorsal and ventral to the neural tube. (H) In puma mutants, sox10+ cells are present medially but appear relatively disorganized as compared with wild-type larvae. (I) In wild-type larvae, foxd3+ cells are widely distributed and are especially abundant within the myotomes (arrow). Some foxd3+ cells also are present around the posterior lateral line nerve, though these cells are fewer in number compared with sox10+ cells at this location. (J) In puma mutants, similar numbers of foxd3+ cells (arrow) are present compared with wild-type. (A, B) 60 μm; (C–F, I, J) 80 μm; (G, H) 80 μm; (K, L) 40 μm. |
|
Defects in peripheral nervous system organization in puma mutants. Staining for nerves and sox10 expression in wild-type (A, C, E) and puma mutant (B, D, F) larvae. All images are cross sections. (A) In wild-type larvae, sox10+ cells (blue arrow) can be observed along peripheral nerves and neurites stained for acetylated tubulin (red arrow), consistent with these cells being glia. (B) In puma mutants, residual sox10+ cells apparent in whole mounts (e.g., Fig. 9F) also can be observed in close association with nerves and neurites. (C) At late stages of pigment pattern metamorphosis in wild-type, the posterior lateral line nerve is cohesive and well organized (red arrow) and is surrounded by slow muscle fibers of the myotome. Blue staining for sox10 can be seen nearby as well. (D) In puma mutants at the same stage, the lateral line nerve appears disorganized and bundles of fibers can be seen both in the normal position (left red arrow) and medially (right red arrow). At this later stage, more sox10+ cells are observed as compared with early metamorphic stages (e.g., Fig. 9B). Arrowhead indicates a melanophore. (E) In a wild-type larva stained only for acetylated tubulin, the lateral line nerve is a solid rod of nerve fibers (arrow). e, epidermis. m, myotome. (F) In puma mutants, the nerve is disorganized and fewer nerve fibers are present (red arrows). Scale bars, (A, B) 10 μm; (C, D) 20 μm; (E, F) 20 μm. |
|
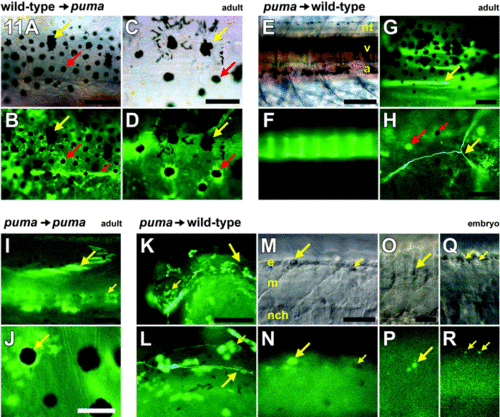
Cell transplantation reveals an essential role for puma in promoting multiple cell lineages during postembryonic development. All images are whole mounts. Red arrows, wild-type cells. Yellow arrows, puma mutant cells. (A–D) Wild-type cells transplanted into puma hosts contributed to a broad array of derivatives (Table 1), including pigment cells, when examined at adult stages. (A, B) Corresponding brightfield and fluorescence views. Many chimeras developed donor-derived GFP+ wild-type pigment cells. Large red arrow, example of a donor-derived wild-type melanophore. Although donor melanophores could be spread widely over the flank, they were typically interspersed with host GFP- puma mutant melanophores, which retained their characteristic large size as compared with wild-type melanophores (yellow arrow). Donor wild-type cells also differentiated into placode-derived tissues and components of the peripheral nervous system: small red arrow, posterior trunk lateral line nerve. (C, D) Corresponding brightfield and fluorescence views. Higher magnification view of a different individual showing donor wild-type GFP+ melanophores (red arrow) adjacent to host puma mutant melanophores (yellow arrow). Note that melanin (contained within melanosomes) remains partially dispersed in the peripheries of the puma mutant melanophores despite treatment with epinephrine, whereas melanin in wild-type melanophores is contracted toward the cell centers. (E–H) puma mutant cells transplanted into wild-type hosts contributed to only a limited array of derivatives (Table 1) when examined at adult stages. (E, F) Corresponding brightfield and fluorescence views showing donor GFP+ puma mutant contribution to vertebrae (v). nt, neural tube; a, aorta. A few wild-type host melanophores are visible in the background above the neural tube and below the aorta. (G) Donor puma mutant cells that differentiated as muscle fibers within the myotome (arrow). (H) In a single chimera, donor puma mutant cells differentiated as a single identifiable neuron (yellow arrow). Fluorescence from adjacent host GFP- xanthophores (large red arrow) is color-shifted relative to GFP fluorescence. Note absence of GFP fluorescence in host melanophore as well (small red arrow). (I, J) puma mutant cells contribute to a broader spectrum of derivatives when transplanted to puma mutant hosts and examined in the adult. (I) Donor GFP+ puma mutant cells can be seen within the myotome (large arrow) and the vertebral column (small arrow). (J) puma mutant cells also contribute to pigment cell lineages in puma mutant hosts, including melanophores (arrow), in contrast to the behavior of these cells in wild-type hosts. (K–R) puma mutant cells are able to produce a wide variety of derivatives at embryonic stages, when transplanted to wild-type embryos. Shown are embryos at 25–27 hpf shortly after melanophores have started to acquire melanin. (K) Head of an embryo showing donor GFP+ puma mutant cells contributing to the eye (small arrow), epidermis, and posterior lateral line nerve (large arrow, enlarged in L). (L) puma mutant donor cells contributing to the migrating posterior lateral line nerve (large arrow) as well as epidermis (small arrow). (M–R) puma mutant cells also are present within the premigratory neural crest and in neural crest migratory pathways, though these cells are often irregularly shaped. (M, N) Corresponding brightfield and fluorescence views of a donor GFP+ puma mutant cell within the epidermis dorsal to the neural crest (large arrow); morphology resembles that of typical zebrafish apoptotic bodies (Parichy et al 1999 and Parichy and Turner 2003b). Small arrow, lightly melanized host wild-type melanophore. (O, P) GFP+ puma mutant cell within the dorsolateral neural crest migratory pathway is irregularly shaped and also resembles typical apoptotic body. (Q, R) Punctate GFP+ puma mutant cells within the neural crest. (A, B) 200 μm; (C, D) 100 μm; (E, F) 200 μm; (G) 100 μm; (H) 60 μm; (I, J) 100 μm; (K, L) 40 μm; (M, N) 40 μm; (O–R) 20 μm. |
Reprinted from Developmental Biology, 256(2), Parichy, D.M., Turner, J.M., and Parker, N.B., Essential role for puma in development of postembryonic neural crest-derived cell lineages in zebrafish, 221-241, Copyright (2003) with permission from Elsevier. Full text @ Dev. Biol.