- Title
-
Function for hedgehog genes in zebrafish retinal development
- Authors
- Stenkamp, D.L., Frey, R.A., Prabhudesai, S.N., and Raymond, P.A.
- Source
- Full text @ Dev. Biol.
|
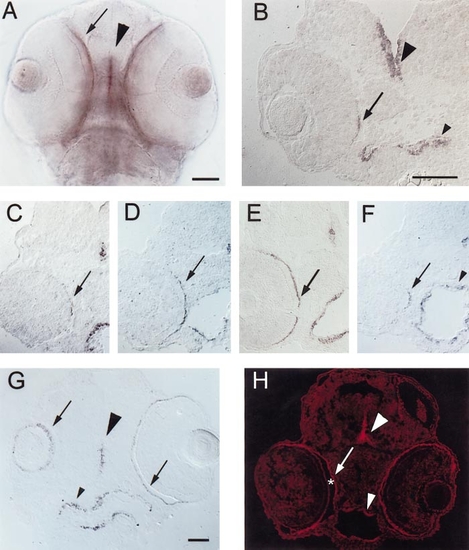
Expression of shh and twhh in the RPE of embryonic zebrafish. (A) PTU-treated, 56-hpf embryo, ventral view of whole mount. shh is expressed at the ventral midline of the developing CNS (arrowhead), in the eye (arrow), and also in the endothelial lining of the foregut (not in focus in the photo but visible as a purple haze below/between the eyes). Scale bar (applies to A, C, D, E, F, and H), 50 μm. (B) Frontal cryosection of a PTU-treated 45-hpf embryo, hybridized with shh cRNA. Expression is evident in the CNS (large arrowhead), in the foregut (small arrowhead), and in a highly restricted pattern of expression in the RPE (arrow). Nearby (posterior) sections passed through the optic nerve (not shown). Scale bar, 50 μm. (C–F) Cryosections of PTU-treated, 54-hpf embryos hybridized with shh (C, E, and F) or twhh (D) cRNA. C, D, and F are sections from the same embryo, C is the most anterior, F is the most posterior. E is a section from a different embryo, but with sectioning depth and orientation corresponding to a region in between D and F. In both embryos, the CNS midline (large arrowheads) and foregut (small arrowheads) are labeled, but the extent to which the signal extends across the RPE varies with the anterior–posterior level of the section. (G) Oblique, frontal cryosection of a PTU-treated 81-hpf embryo, hybridized with shh cRNA; section cuts through the middle of the left eye (right side of photo), but only grazes the ventral surface of the right eye. shh expression is evident in the CNS (large arrowhead), the RPE (arrows), and the foregut (small arrowhead). Scale bar, 50 μm. (H) Cryosection of a PTU-treated, 54-hpf embryo, labeled with a polyclonal rabbit anti-(rat) Hh antibody using indirect cyanine (Cy3) immunofluorescence; large arrowhead indicates labeling of the CNS midline, small arrowhead depicts labeling of the foregut, arrow indicates labeling of the RPE. Some Hh immunoreactivity is present on apical surfaces of developing photoreceptors that are separated from the RPE by the subretinal space (*). |
|
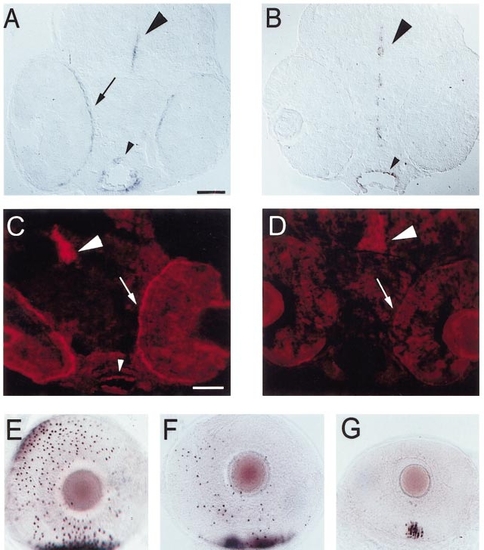
Expression of ptc-2 in embryonic zebrafish. (A) PTU-treated, 45-hpf embryo, hybridized with ptc-2 cRNA. Arrow indicates expression near the foregut. (B) PTU-treated, 55-hpf embryo, hybridized with ptc-2 cRNA. Small arrow indicates expression near foregut; small arrowhead shows expression near CNS midline. Large arrow indicates expression in retinal neuroepithelial cells; large arrowhead shows expression in cells near the optic nerve (*). (C) PTU-treated, 80-hpf embryo, hybridized with ptc-2 cRNA. Small arrow shows expression near foregut; large arrow shows expression in retinal neuroepithelial cells. (D) ptc-2 and zmax RT-PCR products amplified from bodies (b) and eyes (e) of 45-, 48-, 55-, and 80-hpf zebrafish embryos. |
|
Effect of antisense oligo injections on expression of hh genes and on rod recruitment. (A) Cryosection of a PTU-treated embryo injected with a nonsense oligo at 54 hpf, fixed at 56 hpf, and hybridized with shh cRNA. shh is expressed in the RPE (arrow), CNS (large arrowhead), and foregut (small arrowhead). Scale bar (applies to A, B, E, F, and G), 50 μm. (B) Cryosection of an embryo injected with the antisense shh/twhh oligo cocktail, hybridized with shh. shh is expressed in the CNS (large arrowhead) and foregut (small arrowhead), but not in the RPE. (C) Cryosection of a nonsense-injected embryo, labeled with the polyclonal anti-Hh antibody. Hedgehog immunoreactivity is present in the CNS (arrowhead), foregut (small arrowhead), and RPE. Scale bar (applies to C and D), 50 μm. (D) Cryosection of an antisense-injected embryo, labeled with the polyclonal anti-Hh antibody. Hedgehog immunoreactivity is still present in the CNS (arrowhead), but is virtually undetectable in the RPE (arrow indicates position of RPE) and is reduced in the foregut. (E) Eye from a nonsense oligo-injected embryo, fixed at 73 hpf and hybridized with GFrod, lateral view. A dense patch of opsin-expressing rods is present in ventral retina (bottom of photo), and many additional rods have begun to express opsin elsewhere in the eye. (F and G) Eyes from antisense oligo-injected embryos, hybridized with GFrod. In F, there is a dense ventral patch of opsin-expressing rods, but fewer rods are present elsewhere. In G, the ventral patch is considerably smaller and contains fewer rods, and no rods are seen elsewhere in the eye; the eye is also reduced in size compared to C. |
|
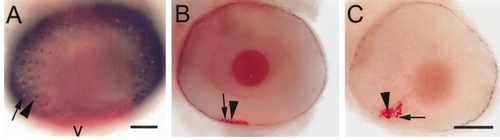
Recruitment of rods and red cones in wild-type and syut4 embryos and in shh/twhh antisense-injected wild-type embryos. Eyes were from 75-hpf embryos, hybridized with GFrod (red color; arrows) and GFred (dark color; arrowheads). (A) Wild-type embryo. Image is a superimposed projection of several focal planes (using the “apply image” function in Adobe PhotoShop), in bright-field and epifluorescence optics. Scale bar (applies to A and B), 50 μm; v, ventral. (B) Eye of embryo injected with shh/twhh antisense oligos. (C) syut4 embryo. B and C are superimposed epifluorescent and bright-field images, but from a single focal plane that contained all labeled photoreceptors. Scale bar in C, 50 μm. |
|
Effect of shh/twhh antisense oligos on expression patterns of other retinal cell-specific markers. (A, C, and E) Sections obtained from embryos injected with the nonsense oligo. (B, D, and F) Sections obtained from embryos injected with the antisense oligo mixture. (A and B) Indirect immunofluorescence with zpr-1 (arrowheads indicate developing cone inner segments). (C and D) Indirect immunofluorescence using the anti-PKC antibody that labels rod bipolar cells. (E and F) Indirect immunofluorescence using the RET1 antibody that labels ganglion cells and a subpopulation of inner nuclear layer cells. l, lens; onl, outer nuclear layer; inl, inner nuclear layer; gcl, ganglion cell layer. Scale bar, 50 μm. |
Reprinted from Developmental Biology, 220(2), Stenkamp, D.L., Frey, R.A., Prabhudesai, S.N., and Raymond, P.A., Function for hedgehog genes in zebrafish retinal development, 238-252, Copyright (2000) with permission from Elsevier. Full text @ Dev. Biol.