- Title
-
The zebrafish colourless gene regulates development of non-ectomesenchymal neural crest derivatives
- Authors
- Kelsh, R.N. and Eisen, J.S.
- Source
- Full text @ Development
|
Melanoblasts are abnormal in cls- embryos. PHENOTYPE:
|
|
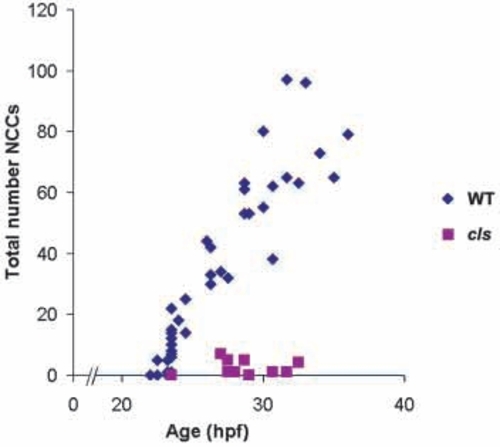
Lateral migration path neural crest cells are severely reduced in cls-/- embryos from the earliest stages. Neural crest cell counts of each embryo are plotted as a single symbol (wild-type = blue, n=39; cls- = pink, n
|
|
Enteric neurons are extremely reduced in cls- larvae. Transverse sections of posterior trunk of (A) wild-type larva at 7 dpf stained for the anti-Hu epitope reveal numerous Hu-positive neurons (arrowhead) around the hindgut (hg). A sibling cls- larva (B) lacks these cells. In whole-mount hindguts, dissected at 5 dpf, cls- larvae have only about 13% as many enteric neurons (28.4±10.9; n=7), compared to wild types (212±20.9; n=3). f, ventral fin; m, myotome; me, melanophore. Scale bar, 35 μm. |
|
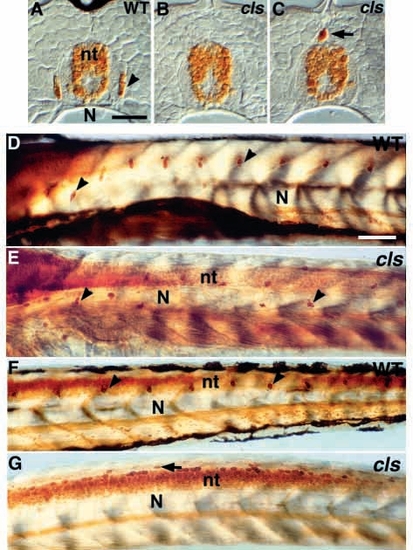
DRG sensory neurons are disrupted in cls- larvae. (A-C) Transverse section of dorsal tail of (A) wild-type larva at 7 dpf stained with anti-Hu antibody reveals small clusters of Hu-positive neurons (arrowhead) in DRG. These are usually absent from the tail of cls- homozygotes (B). cls- larvae have an increased frequency of extramedullary cells dorsal to the neural tube (nt; arrow in C and G). (D-G) Lateral views of whole-mount larvae at 3 dpf stained with anti-Hu antibody reveal a segmental pattern of DRGs (arrowheads) throughout the trunk (D) and tail (F), but these cells are reduced and misplaced in the trunk (E) and absent from the tail (G) of cls- larvae. N, notochord; sc, spinal cord. Scale bar, 25 μm (A-C) and 75 μm (D-G). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cranial sensory neurons are unaffected in cls- embryos. Lateral views of whole-mount wild-type (A) and cls- (B) 24 hpf embryos stained with zn-12 and anti-acetylated tubulin antibodies. e, eye; tri, trigeminal ganglion; ac, acoustic ganglion; allg, anterior lateral line ganglion; pllg, posterior lateral line ganglion; plln, posterior lateral line nerve. Scale bar, 60 μm. EXPRESSION / LABELING:
|
|
Putative cranial glia are strongly decreased in cls- embryos. Lateral views of 24 hpf wild-type (A,C) and cls- (B,D) embryos labeled for fkd6 expression (purple) and Hu epitope (orange, A,B only) show tight association of fkd6 labeling with cranial ganglia. The number of fkd6-positive cells associated with cranial ganglia (A,B) and posterior lateral line nerve (C,D) is markedly reduced in cls- embryos. In A and B, fkd6 labeling was stopped early to prevent masking of the anti-Hu staining. Abbreviations as Fig. 5. Scale bar, 40 μm. EXPRESSION / LABELING:
|
|
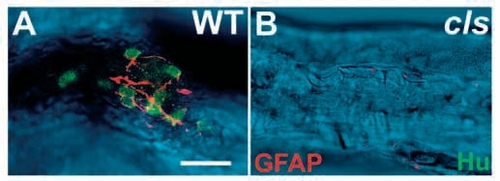
GFAP-positive enteric glia are largely absent in cls- larvae. Dissected 5 dpf hindgut preparations stained with anti-Hu (green) and anti-GFAP (red) antibodies clearly reveal the enteric nervous system in the wild type (A) and its essential absence from cls- siblings (B). Confocal sections of the fluorescent labeling are superimposed on a DIC image of the gut. Scale bar, 20 μm. |
|
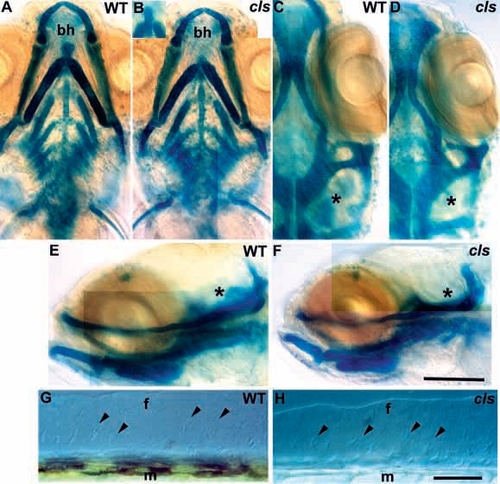
Ectomesenchymal crest derivatives are essentially normal in cls- embryos. Alcian green stained craniofacial skeletons of 5 dpf wild-type (A,C,E) and cls- (B,D,F) larvae are shown in ventral (A,B), dorsal (C,D) and lateral (E,F) views. Mutant craniofacial skeletons are barely distinguishable from wild type, although development is slightly retarded (compare angle of main arch elements in A and B) and the basihyal cartilage is variable (bh in A and B, also inset in B). Ventral (C,D) and lateral (E,F) views show slight differences in the cartilages associated with the developing inner ear (asterisk) which remains small in cls-. Median fin mesenchyme (arrowheads) of the dorsal fin in lateral view with Nomarski optics in 5 dpf wild-type (G) and cls- (H) larvae; the number and position of cells is identical. f, medial fin; m, muscle blocks. Scale bar, 100 μm (A-F) and 50 μm (G,H). |
|
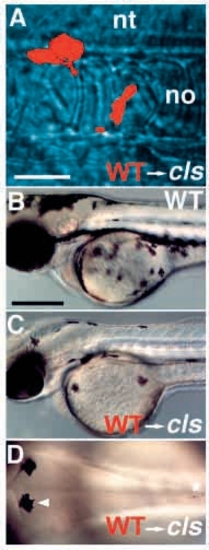
Wild-type→cls- chimaeric embryos show enhanced numbers of pigment cells. Wild-type donor cells labeled with rhodamine dextran (red) and transplanted into an unlabeled cls- host embryo at shield stage frequently contributed to neural crest. Three cells are seen here migrating past the notochord (no) at 24 hpf (A). Some chimaeric cls- embryos show many (C) or few (D) melanophores by 2 dpf; all such embryos are clearly distinguishable from wild types (B). (D) All these melanophores have a wild-type appearance [compare wild-type melanophore (arrowhead) and mutant melanophore (asterisk)]. A-C, lateral views; D, dorsal view of posterior head. nt, neural tube. Scale bars, 50 μm (A) and 250 μm (B-D). |
|
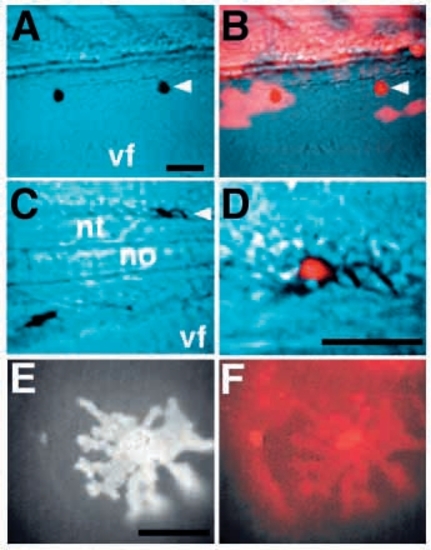
cls acts cell-autonomously in all three pigment cell fates. Chimaeric embryos generated by transplanting rhodamine-dextran labeled wild-type cells into cls- hosts show ectopic iridophores (A), melanophores (C) and xanthophores (E). These chromatophores are entirely derived from the labeled donor (red; B,D,F). A and C are views with Nomarski optics, B and D are superimposed Nomarski and fluorescence views. A labeled xanthophore shows its characteristic autofluorescence (E) and rhodamine fluorescence (F). vf, ventral fin; nt, neural tube; no, notochord. Scale bars, 50 μm (A-D) and 25 μm (E,F). |