- Title
-
nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate
- Authors
- Lister, J.A., Robertson, C.P., Lepage, T., Johnson, S.L., and Raible, D.W.
- Source
- Full text @ Development
|
The nacre mutant. Lateral (A) and dorsal (B) views of wildtype (top) and nacre (bottom) larvae at 3 days postfertilization. nacre homozygotes are missing all neural-crest-derived melanophores, but pigmentation of the eye is normal. (C) Tail iridophores, viewed with epi-illumination. nacre mutants (bottom) have increased numbers of iridophores, including many in the tail fin. (D) Xanthophore pigmentation, viewed under UV light, is slightly reduced in nacre mutants (bottom). All visible fluorescence is due to xanthophores, while the dark patches in the top panel are melanophores. Scale bar is approximately 500 μm in A-C, 100 μm in D. PHENOTYPE:
|
|
Pigmentation in wild-type and nacre adults. (A) The melanophore defect persists throughout development. nacre adults display widespread iridophore pigmentation, primarily on the ventral torso, and variable xanthophore pigmentation, but no melanophore stripes. Arrowheads indicate a faint iridophore stripe. (B) Melanophores are occasionally observed in adult fins. Shown here, anal fins of typical wild-type (left) and nacre (center) adults, and from a nacre animal with a patch of melanophores (right). Scale bar is approximately 500 mm in A, 250 mm in B. PHENOTYPE:
|
|
Transplants suggest that nacre functions cell-autonomously. Cells from a wild-type embryo labeled with a lineage tracer were transplanted to a nacre host. The arrows indicate a pigmented cell with melanophore morphology (top panel, Nomarski optics) which contains the rhodamine-dextran lineage tracer (bottom panel, fluorescence) demonstrating its donor embryo origin. Scale bar, 100 μm. |
|
Expression of melanoblast markers is absent from nacre-/- neural crest. Whole-mount in situ hybridization was performed on 23 hpf embryos with probes for trp2(A) and c-kit (B). Expression of trp2 is strong in the eye of nacre mutants (arrow) but absent from the neural crest (arrowheads). Likewise, nacre-/- embryos show normal expression of the receptor tyrosine kinase c-kit in the intermediate cell mass (arrow) and elsewhere but little or no expression in presumptive melanoblasts (arrowheads). Scale bar, 250 μm. EXPRESSION / LABELING:
|
|
Expression of the zebrafish Mitf-related gene during development. Wholemount in situ hybridization was performed with an antisense probe to 3A.1. (A) Expression (arrowheads) in the caudal margin of the eye and in head neural crest of an 18-somite (18 hpf) embryo. Expression is also detectable in a few cells in the trunk neural crest at this stage (not shown). 3A.1 expression expands in the eye and progresses in the head and trunk in a rostral to caudal manner as seen in 21 hpf (B) and 23 hpf (C) embryos. Migratory cells can clearly be seen by the latter timepoint. (D) 3A.1 (blue, top panel) and the melanoblast marker trp2 (red, bottom panel) are coexpressed in migrating melanoblasts of 24 hpf embryos. (E) Expression of 3A.1 is reduced in nacre mutants. The top panel shows a closeup of the 23 hpf embryo from (C). 3A.1-expressing cells can be seen on migratory paths at each somite level. Expression is much reduced and few cells have migrated away from the neural tube in a nacre mutant embryo at the same stage (bottom panel). (F) Reduced 3A.1 expression is still clearly evident at 30 hpf in a comparison of albino (top) and nacre (bottom) embryos. Scale bar: A, 100 μm; B,C, 200 μm; D,E, 50 μm; F, 250 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Rescue of melanophore development by ectopic expression of wild-type nacre. Homozygous nacw2 embryos were injected with a construct bearing wild-type nacre under the control of the zebrafish heat-shock promoter. (A-C) dorsal views of representative examples are shown at approximately 72 hpf. Heat shock administered between 12 and 20 hpf restored melanophore development to varying degrees, ranging from quite extensive rescue (B) to just a few cells (C). Additionally, pigmented cells of unusual morphology were frequently observed in uncharacteristic locations, as seen here on the ventral yolk of a 27 hpf embryo (D, arrowheads). Scale bar, A-C, 500 μm; D, 200 μm. |
|
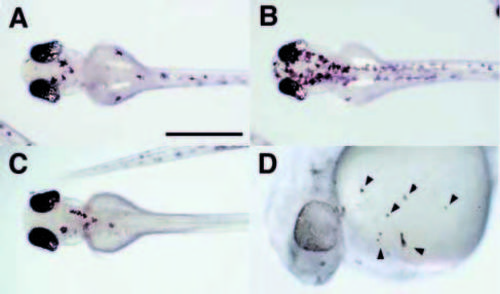
Misexpression of nacre induces ectopic melanophores and disrupts eye development. (A,B) Wild-type embryos injected with nacre RNA at the 1- to 4-cell stage are shown at approximately 30 hpf. Embryos were frequently observed that displayed large patches of melanophores with abnormal morphology, as well as patches of similar cells in uncharacteristic locations such as the ventral yolk ball (A, arrows). Arrowheads indicate normal melanophores. Disorganization of one or both eyes in otherwise normal embryos was frequently observed (B). (C-F) Ectopic expression of nacre induces ectopic expression of trp2. Embryos were injected with pHSMT3A. 1-I219F (C) or pHS-MT3A.1 (D) and heat shocked from 10-12 hpf, then fixed at 14 hpf and processed for in situ hybridization with probes for melanophore marker trp2 (blue) and the neural crest marker fkd6 (orange). Widespread trp2-positive cells are observed in (D) prior to neural crest emigration. Injection of wild-type (F) but not mutant (E) nacre RNA expands the domain of trp2 expression in the optic primordium of some embryos at 18 hpf. (G,H) Examples of disrupted eye morphologies in frontal sections of 72 hpf embryos. The embryo in G has one approximately normal eye on the left, and a second laminated structure with a small amount of RPE on the right (arrows). The eyes in the embryo in H are of reduced size and show disorganization and irregularities in the RPE. Arrowheads, ectopic pigment cells. Scale bar, A,B, 250 μm; C-F, 300 μm; G,H, 100 μm. |