- Title
-
An important developmental role for oligosaccharides during early embryogenesis of cyprinid fish
- Authors
- Bakkers, J., Semino, C.E., Stroband, H., Kijne, J.W., Robbins, P.W., and Spaink, H.P.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
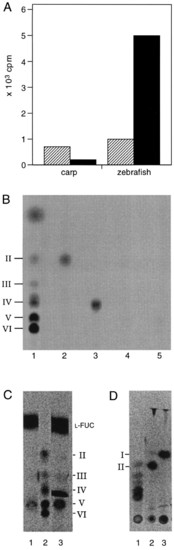
Radiolabeling of gastrulation-specific metabolites. Extracts of zebrafish or carp embryos from the gastrulation stage were incubated in the presence of radiolabeled or unlabeled UDP-GlcNAc (see Materials and Methods). (A) Incorporation of UDP-[U14C]GlcNAc into HPLC fractions with retention times similar to chitin tetraose (striped box) and chitin pentaose (filled box). In the incubations where unlabeled UDP-GlcNAc was used, the equivalent fractions were incubated with the NodZ protein in the presence of GDP-[U-14C]fucose. By using this assay it is possible to specifically detect chitin oligosaccharides at concentrations as low as 1 picomol (data not shown). The pooled fractions were used for chitinase and chitobiase treatments and separated on TLC (B-D). (B) Radiolabeling of metabolites obtained from carp embryos and separated by HPLC, using the NodZ transfucosylation assay. Lanes: 1, fucosylated chitin oligosaccharide standards (as described in ref. 4); 2 and 3, HPLC fractions with retention times similar to chitin tetraose after transfucosylation, incubated with chitinase (lane 2) or without chitinase (lane 3); 4 and 5, HPLC fractions with retention times similar to chitin pentaose after transfucosylation, incubated with chitinase (lane 4) or without chitinase (lane 5). (C and D) Radiolabeling of metabolites obtained from zebrafish embryos using the NodZ transfucosylation assay. Fucosylated chitin oligosaccharide standards (C, lane 2; and D, lane 1); HPLC fractions with retention times similar to chitin pentaose after transfucosylation {C, lanes 1 and 3 (without removing free GDP-[U-14C]fucose)} incubated with chitinase (D, lane 2), or incubated with chitinase and chitobiase (from Streptomyces griseus, Sigma) (D, lane 3). |
|
Results of microinjection experiments in zebrafish. All embryos shown are 48 h old and represent typical examples. (A) Embryos injected with DG42 antiserum. Sixty-one percent of the embryos (n = 288) were consistently and reproducibly affected in the formation of the trunk and tail. (B) Embryos injected with NodZ protein. Sixty-nine percent of the injected embryos (n = 106) show defects similar to those observed after injection of the DG42 antiserum. As a control, embryos were injected with an identical preparation of NodZ protein inactivated prior to injection by boiling for 5 min. Sixteen percent of these controls (n = 98) were affected, although the defects observed are nonspecific and do not resemble those seen when injecting the active protein (data not shown). (C) Control embryos injected with rabbit preimmune serum. Five percent of control embryos (n = 116) were affected by the injection procedure, but the observed defects were not specific. (Bars = 250 μm.) |