- Title
-
Magnetic resonance microscopy and spectroscopy reveal kinetics of cryoprotectant permeation in a multicompartmental biological system
- Authors
- Hagedorn, M., Hsu, E.W., Pilatus, U., Wildt, D.E., Rall, W.R., and Blackband, S.J.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
Image of a six somite zebrafish embryo that identifies the major compartments (yolk and blastoderm). Although the YSL would not be visible in this image, its position has been drawn for clarity. |
|
False-color enhanced MR images of water in six somite dechorionated zebrafish embryos using a modified spin echo sequence (11) (128 x 128 pixels, 17 x 17 x 100 μm resolution, 3 sec repetition time; TR, the time between successive excitation pulses, two averages). Cross-sectional images were obtained through the center of embryos [diameter of six somite ca. 800 μm (6)] immersed in embryo medium held in a 1-mm glass capillary. (A) A T2-weighted image obtained using a relatively long echo time (TE, the delay between excitation and formation of the spin-echo = 55 msec) so that components with a longer T2 appear bright. This image depicted a relatively dark embryo surrounded by embryo medium (bright yellow region). The yolk appeared at the center of the embryo (dark red) with the blastoderm projecting on either side (shown in red). (B) A diffusion-weighted image obtained by applying a pair of gradient pulses (50 G/cm in magnitude, 3 msec in duration, and 3.5 msec pulse separation) around the 180° (nonslice selective) refocusing rf pulse. Spins that have diffused during the gradient pair acquire net phase shifts, resulting in a signal loss through interference. This signal loss increases with increased diffusion coefficients. These images of a zebrafish embryo reveal a relatively bright blastoderm (shown in yellow and red), dark yolk, and dark embryo medium (shown in red and black). This image indicated a lower water diffusion coefficient in the blastoderm than in the yolk and the surrounding medium. Embryos were dechorionated as previously described (12), and figures were false-color enhanced using a standard color palette from a computer graphics program (IMAGE program from National Institutes of Health). |
|
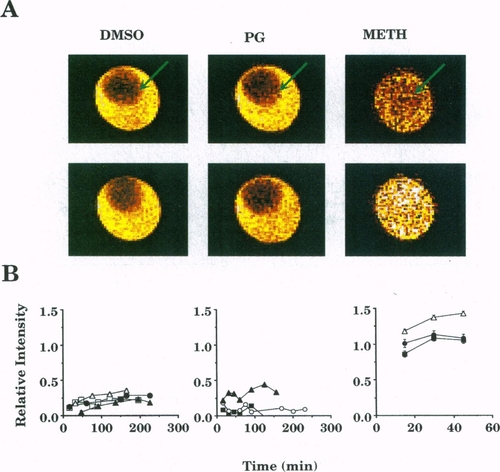
CSIs of three somite dechorionated zebrafish embryos immersed in a cryoprotectant and the relative signal intensities of these images. A modified spin-echo imaging sequence was used in which the 90° rf excitation pulse was chemical shift selective (see Fig. 3). Image slice was selected by the 180° refocusing rf pulse. In the images, the cryoprotectant was bright in the medium surrounding the embryo (arrow). A bright embryo indicated permeation of the cryoprotectant. (A Top) First chemical-shift images depict embryos after immersion in 2 M DMSO, 2 M PG, or 2.25 M METH in embryo medium for 15 min. Images of the DMSO- and PG-treated embryos were depicted as dark red circles (arrows), indicating little or no permeation. METH indicated permeation within the first 15 min (arrow indicates position of permeated embryo in capillary). Image in-plane resolution = 34 x 34 μm. (A Bottom) Subsequent images depicted embryos after 2 h exposure to 2 M DMSO and 2 M PG and 45 min to 2.25 M METH. Images of the DMSO- and PG-treated embryos remained dark at 2 h, indicating little or no permeation. Image in-plane resolution = 40 x 40 μm, slice thickness 300 or 600 μm, TR = 3 sec, TE = 31 msec, image matrix = 128 x 128 with two averages. Images were false-colored as described in Fig. 2. During postprocessing and after analysis, slightly different scaling factors were applied to the images to enhance contrast. (B) Plots of the relative signal intensities (normalized to that of the surrounding embryo medium) for embryos as a function of exposure time to the cryoprotectant solution. Each point represented the ratio of the signal intensities averaged over corresponding regions-of-interest (i.e., ca. 200 pixels in both the yolk and medium). Regions-of-interest templates were obtained for the embryos using image segmentation techniques. To minimize in-plane volume averaging with the embryo medium, before averaging templates were morphologically eroded until the pixel intensity over the template exhibited a Gaussian distribution (11). Average signal intensities were obtained and normalized to those of the embryo medium. METH permeated the embryos within 15 min (n = 3), whereas little or no DMSO or PG permeation was observed over 2.5 h (n = 4 and 3, respectively). After 45 min, the METH image indicated a weak increase in the signal intensity, suggesting long-term, time-dependent METH permeation. The METH values are larger than one possibly due to the T1 and/or diffusion weighting of the intraembryonic METH signal (see text for details). Standard error bars were obscured by the symbols in most cases, and each symbol type corresponded to an individual experiment. |