- Title
-
Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation
- Authors
- Chen, J.N. and Fishman, M.C.
- Source
- Full text @ Development
|
(A-F) The expression pattern of zebrafish Nkx2.5, revealed by whole-mount in situ hybridization, correlates with the position of cardiac precursors. (A) The Nkx2.5-expressing cells (arrows) are located on the left and the right of the midline in part of the lateral plate, at the 10-somite stage. (B) Transverse section reveals that the ,em>Nkx2.5-expressing cells organize in tubes on the right and the left of the midline (arrows) ventral to the neural tube at the 18-somite stage. (C) Starting at 20-somite stage, the two tubes move toward the midline and begin to fuse at the caudal side (arrows). (D) The fusion of the primitive heart tube is complete by the 24-somite stage, shown as a ring-like structure of zebrafish Nkx2.5-expressing cells at the midline (arrow). (E) By 26 hours postfertilization (hpf), the heart moves to the left side of the embryo (arrow). (F) Nkx2.5 expression is restricted to the heart in a 48 hpf embryo (arrow). Anterior to the left in all but B. A, C, D are dorsal views. E is dorsolateral view from the left side; F is left lateral view. hd, head; nt, neural tube. EXPRESSION / LABELING:
|
|
(A-C) Nkx2.5 expression can be detected at the onset of gastrulation. (A) It is restricted to the ventral margin of the embryo at 50% epiboly. The yolk is scrapped off intentionally to visualize the gradient of Nkx2.5 and the arrowheads point to the edge of the embryo. B shows goosecoid expression (arrowhead) as a dorsal reference. (C) The Nkx2.5- expressing cells migrate in the hypoblast, the term used to refer to deeper cell layers in zebrafish gastrulation. e, epiblast; h, hypoblast; y, yolk; An, animal pole; D, dorsal. EXPRESSION / LABELING:
|
|
Injection of Nkx2.5 mRNA (100 pg) causes an enlarged heart. The atrium of (A) lacZ-injected and (B) Nkx2.5- injected embryos (arrows point to the border of the atrium). Embryos are oriented with dorsal to the top and anterior to the left. |
|
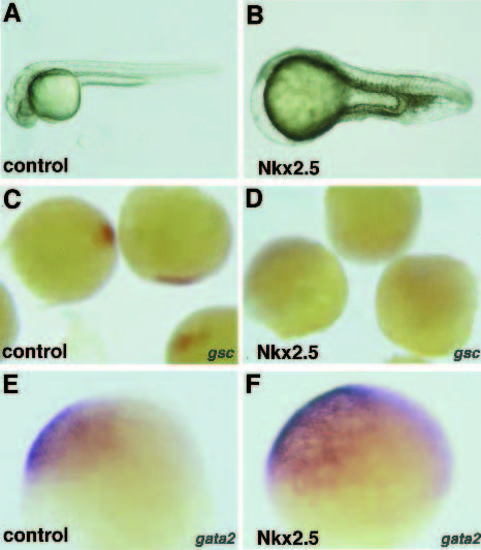
Dorsoventral axial defects are caused by higher-dose Nkx2.5 RNA injection. (A) Control embryo after 1 day of development. (B) A severely affected zebrafish embryo after NKx2.5 injection. The head and eyes are missing, notochord is diminished and the somites are fused. (C-F) Effects on the dorsoventral axis are evident during gastrulation, with (C,D) reduction of the expression of the dorsal marker, goosecoid, and (E,F) expansion of the domain of a ventral lateral marker, gata2 . C and E show embryos injected with 250 pg lacZ mRNA, probed with gsc and gata2 respectively. D and F show embryos injected with 250 pg zebrafish Nkx2.5 RNA, and probed with gsc and gata2, respectively. |
|
Injection of higher doses of Nkx2.5 mRNA (250 pg) causes ectopic myosin heavy chain expression. In the chest region, this is evident as (A) two beating hearts (arrows), shown in their two pericardial sacs or (B) with MF20 labeling (arrows). Elsewhere, patches of ectopic MF20 immunoreactivity may be found (C,D) in the brain (arrows) or (E,F) on the yolk (arrows). The other MF20 immunoreactive structure is the heart (arrowhead). Embryos are oriented with dorsal to the top and anterior to the left in A,D and F. B is a ventral view and C and E are dorsal views of the embryo. hd, head. |
|
Transplanted Nkx2.5-expressing cells express cardiac markers in ectopic locations. (A) Schematic of method used to coinject zebrafish donor embryos at the 1- to 2-cell stage with Nkx2.5 RNA and tetramethylrhodamine dextran as described by Lee et al. (1994). A few cells were transplanted to the animal pole of an unlabelled host. They subsequently populated the brain or trunk. B, C and D are confocal microscope images of the brain region shown in A. (B) Donor cells labeled by tetramethylrhodamine dextran. (C) A subset of cells in the same region are reactive to the atrial-specific antibody, S46. (D) A superimposed image of B and C, showing that all S46 immunoreactive cells are also rhodaminelabeled, and hence derived from the donor. |
|
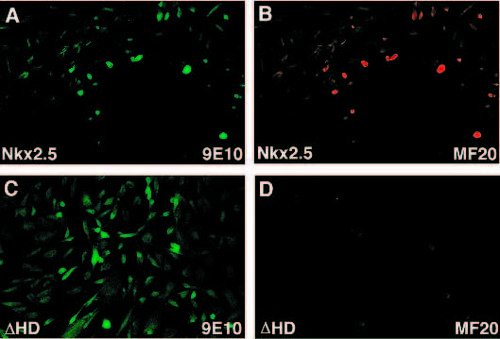
Nkx2.5 activates cardiac genes in fibroblasts. A and B are images of the same field after transfection with myc-tagged Nkx2.5. C and D are of the same field after transfection with myc-tagged Nkx2.5 missing the homeodomain. (A,B) All of the cells expressing the product of the transfected gene, as revealed by immunoreactivity for the myc tag (A), are also immunoreactive for MF20 (B). (C) The myc-tagged construct with Nkx2.5 missing the homeodomain transfects cells equally well, such that they become immunoreactive for 9E10 but (D) no cells express MF20. |