- Title
-
Acitretin mitigates uroporphyrin-induced bone defects in congenital erythropoietic porphyria models
- Authors
- Bragazzi Cunha, J., Elenbaas, J.S., Maitra, D., Kuo, N., Azuero-Dajud, R., Ferguson, A.C., Griffin, M.S., Lentz, S.I., Shavit, J.A., Omary, M.B.
- Source
- Full text @ Sci. Rep.
|
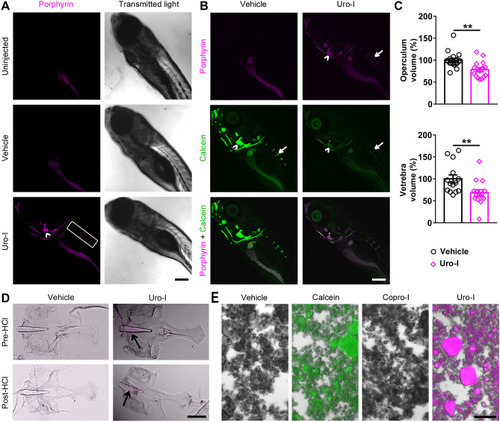
Zebrafish model of CEP develops bone phenotype resembling human disease. ( PHENOTYPE:
|
|
Acitretin mitigates CEP bone phenotype in zebrafish. (A) 6dpf larvae were injected with uro-I and transferred to medium containing acitretin or DMSO. At 7dpf larvae were injected with calcein and imaged by confocal microscopy. Quantification of porphyrin fluorescence (B), porphyrin excretion (C) and operculum volume (D) from experiment in (A). Symbols represent individual larvae (12–25/group) from 3–4 independent experiments. (E) 6dpf larvae were injected with uro-I. At 7dpf they were transferred to medium containing acitretin or DMSO. At 8dpf larvae were injected with calcein and imaged by confocal microscopy. Quantification of porphyrin fluorescence (F), porphyrin excretion (G) and operculum volume (H) from experiment in (E). Arrowhead-operculum; box-vertebrae (A, E). Symbols represent individual larvae (18–64/group) from 3–4 independent experiments. (I-K) Larvae were treated as in (A) with the indicated retinoid or DMSO and porphyrin fluorescence (I), porphyrin excretion (J) and operculum volume (K) were assayed. Bone volume was normalized to DMSO-treated larvae set to 100%, (D, H, K). Porphyrin excretion was normalized to DMSO-treated larvae set to 100%, (C, G, J). Symbols represent individual larvae (7–44/group) from 2–4 independent experiments. Scale bars: 200 µm. Three-dimensional image reconstruction (A, E) was performed using Imaris 3D software v7.7 (http://imaris.oxinst.com/). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. PHENOTYPE:
|
|
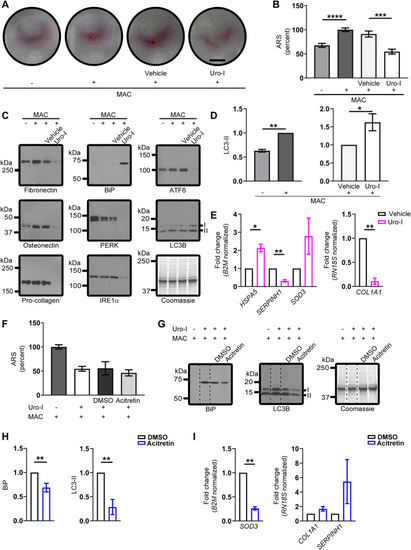
Saos-2 cells mimic CEP zebrafish model. ( |
|
Proposed model of CEP pathogenesis. UROS inhibition leads to production of uro/copro-I mostly in erythrocytes and liver, which is transported through blood to the bones. Uro-I causes bone damage by binding to hydroxyapatite, causing oxidative and ER stress, protein aggregation and stalled autophagy. Acitretin partially rescues uro-I-induced bone damage by reducing oxidative and ER stress and restoring autophagic flux. |