- Title
-
Diversity and robustness of bone morphogenetic protein pattern formation
- Authors
- Madamanchi, A., Mullins, M.C., Umulis, D.M.
- Source
- Full text @ Development
|
|
|
|
|
|
|
|
|
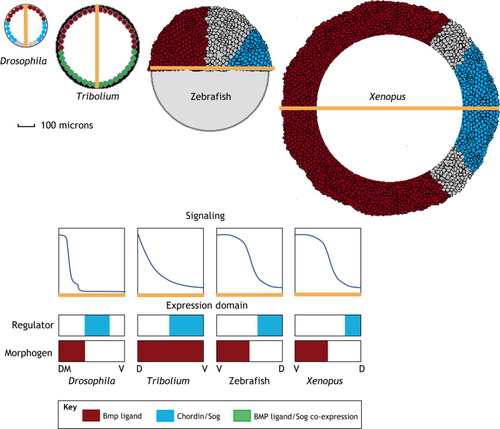
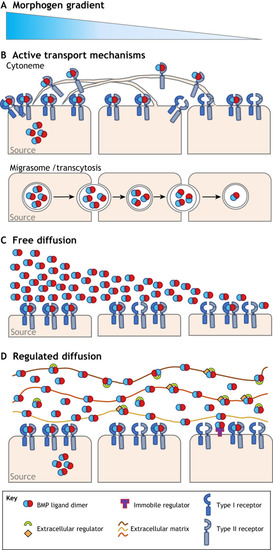
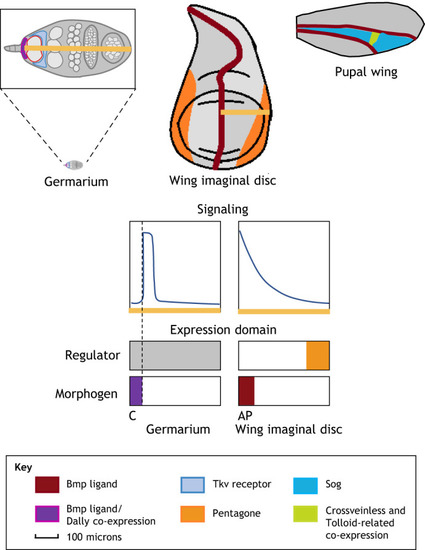
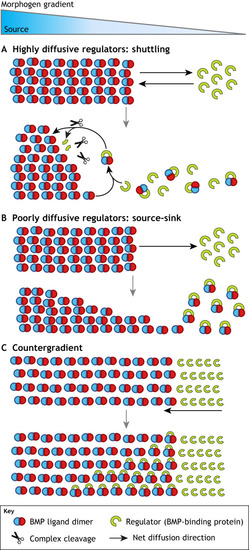
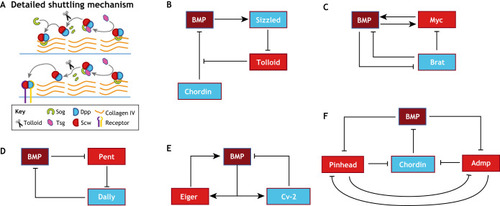
(A) BMP/Dpp shuttling as observed in Drosophila DV patterning. Sog binds Dpp/Scw ligand heterodimer and forms a Type IV Collagen bound complex that prevents signaling in areas of high Sog concentration. Tsg disrupts Sog-Dpp/Scw binding to Collagen and enables diffusion. Tsg also acts as a scaffold to promote Tolloid-mediated Sog cleavage and Dpp/Scw liberation. In areas with high Sog levels, the liberated ligand heterodimer typically reforms a Sog complex and begins another round of shuttling. In high Tolloid and low Sog levels areas, the ligand heterodimer is free to signal. Iterative rounds of complex formation and cleavage have the effect of moving ligand away from Sog expression, leading to a concentrated high peak at the dorsal midline. (B-F) Network diagrams of extracellular BMP regulation in diverse contexts. (B) BMP influences its own extracellular regulation in embryonic axis formation. BMP signaling leads to upregulation of the ventral protein Sizzled, which competitively inhibits Tolloid and prevents Tolloid-mediated Chordin cleavage. (C) In the Drosophila germarium, downstream BMP signaling target, Myc, provides positive feedback by upregulating BMP ligand uptake. Brat creates a bistable switch for differentiation by inhibiting BMP signal transduction and Myc activity. (D) In the Drosophila wing disc, Pent, a secreted factor that is negatively regulated by BMP signaling, supports BMP signaling by directing the internalization of Dally, a negative regulator of BMP signaling. (E) In the Drosophila embryo, downstream BMP signaling products Eiger and Cv-2 provide positive and negative feedback, respectively, to BMP signaling. These feedback mechanisms act to fine tune the BMP gradient and confer spatial bistability. (F) In zebrafish, Pinhead and Admp both act to support BMP signaling by promoting Chordin degradation. Pinhead and Admp are both downregulated by each other and by BMP signaling. The reciprocal repression circuit of Admp and Pinhead provides robustness in BMP gradient formation. |