- Title
-
Unbiased Identification of Angiogenin as an Endogenous Antimicrobial Protein With Activity Against Virulent Mycobacterium tuberculosis
- Authors
- Noschka, R., Gerbl, F., Löffler, F., Kubis, J., Rodríguez, A.A., Mayer, D., Grieshober, M., Holch, A., Raasholm, M., Forssmann, W.G., Spellerberg, B., Wiese, S., Weidinger, G., Ständker, L., Stenger, S.
- Source
- Full text @ Front Microbiol
|
Screening a hemofiltrate library for activity against extracellular |
|
Activity of synthetic Angiogenin against extracellular |
|
Effect of Angiogenin on macrophage viability and |
|
|
|
Antimycobacterial activity of Angie1 against extracellular |
|
Effect of Angie1 on macrophage viability and multiplication of intracellular |
|
Antimicrobial effect of Angie1 against fast-growing bacteria. About 2 × 107 bacteria were seeded into a petri dish containing agarose. Angie1 (Core Facility Peptidomics) was given into cavities in the agarose. After 3 h, plates were overlaid with agarose and after 18 h of incubation, inhibition zones were measured for |
|
Half-life of Angie1 in human serum. Human serum was spiked with Angie1 (10 μM) and aliquots of the samples were harvested at indicated time points. The amount of peptide was determined by mass spectrometry. The graph shows the amount of peptide (%) of the initial inoculum ± SD of three individual measurements. |
|
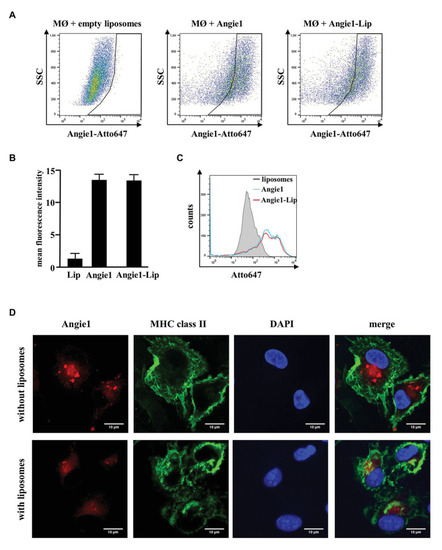
Uptake of Angie1 and Angie1-lip in macrophages. (A) Macrophages were incubated overnight with Atto647N-labeled Angie1 or Angie1-lip (both 54 μM, PSL Heidelberg). After 18 h, cells were harvested and analyzed by flow cytometry. Dot plots show one representative donor of three for each group. (B) The graph shows the mean fluorescence intensity (FI) ± SD of Atto647-positive cells for empty liposomes, Angie1 and Angie1-Lip of three independent donors. Statistical analysis was performed using a non-parametric Wilcoxon-Rank Test for paired samples. (C) The histogram shows the counts of Atto647-positive populations for empty liposomes, Angie1 and Angie1-Lip for one representative donor of three donors. (D) Macrophages were incubated with Angie1-Atto647N (upper panels) or Angie1-Atto647N-Lip (lower panels). After 18 h, cells were stained for MHC class II. Cell nuclei were stained with DAPI. Images were acquired using an inverted laser scanning confocal microscope (Zeiss LSM 710). Depicted images show representative area of one out of three donors with similar result. |
|
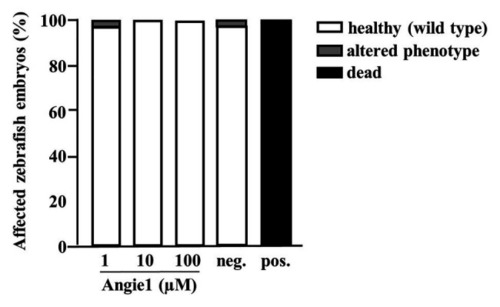
Angie1 is not toxic to zebrafish embryos. Zebrafish embryos were scored for mortality or altered phenotypes at 48 h post fertilization (hpf) after exposure for 24 h to Angie1 at the indicated concentrations or the negative control (PBS) or positive control [NRC-03 antimicrobial peptide, (AMP)]. Altered phenotypes include necrosis and non-lethal lysis (cytotoxicity), heart edema, reduced or absent circulation (cardiotoxicity), delayed development or malformations (developmental toxicity), and reduced or absent touch escape response (neurotoxicity). Note that Angie1 caused no significant toxicity. |