- Title
-
Studying molecular interactions in the intact organism: fluorescence correlation spectroscopy in the living zebrafish embryo
- Authors
- Dawes, M.L., Soeller, C., Scholpp, S.
- Source
- Full text @ Histochem. Cell Biol.
|
Overview of FCS set-up and zebrafish measurements. |
|
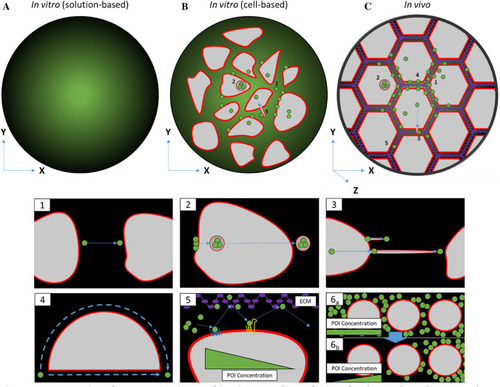
Comparison between in vitro and in vivo sample analysis. |
|
mRNA injection time determines distribution of fluorophore. |