- Title
-
Mitochondrial Calcium Uniporter (MCU) deficiency reveals an alternate path for Ca2+ uptake in photoreceptor mitochondria
- Authors
- Bisbach, C.M., Hutto, R.A., Poria, D., Cleghorn, W.M., Abbas, F., Vinberg, F., Kefalov, V.J., Hurley, J.B., Brockerhoff, S.E.
- Source
- Full text @ Sci. Rep.
|
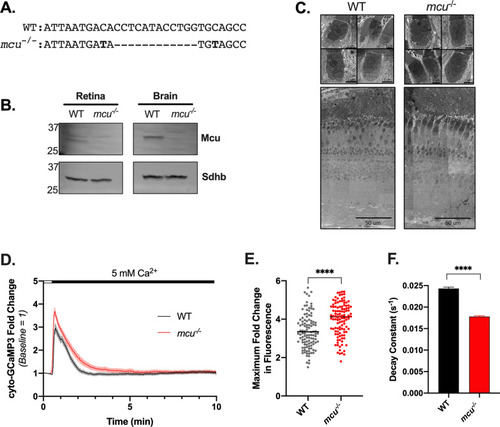
Mcu contributes to clearance of cytosolic Ca2+ in zebrafish cones. ( |
|
Mitochondrial Ca2+ uptake in cones from global PHENOTYPE:
|
|
Retinas from global PHENOTYPE:
|
|
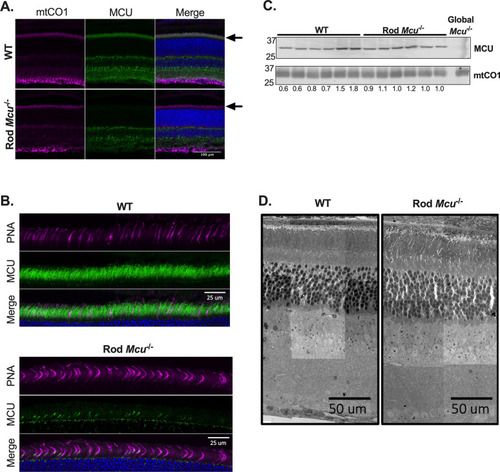
Rods express low levels of MCU. ( |
|
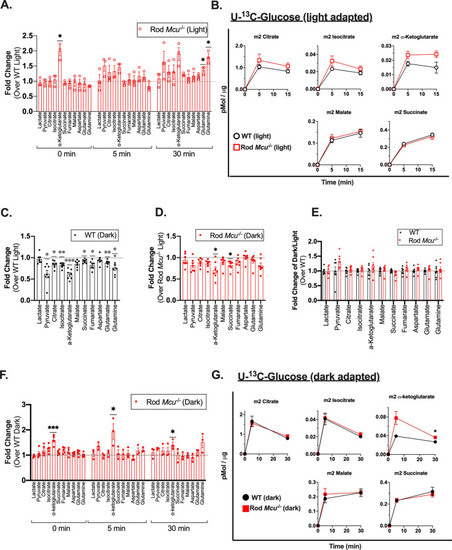
Loss of MCU leads to a buildup of α-ketoglutarate in Rod |
|
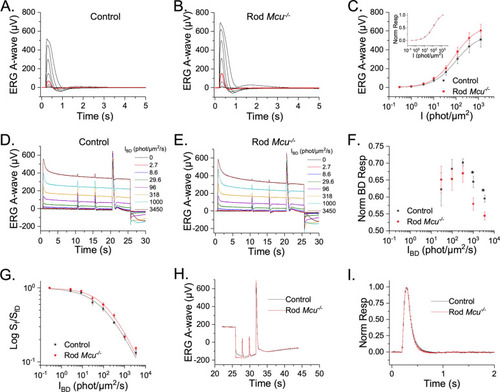
Mouse rods lacking Ca2+ uptake through MCU exhibit normal photoresponse. ( |
|
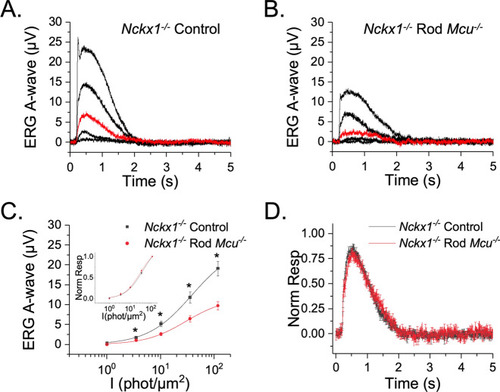
MCU-mediated mitochondrial Ca2+ uptake does not modulate the photoresponses in Nckx1-/- mice. ( |