- Title
-
Fisetin protects against cardiac cell death through reduction of ROS production and caspases activity
- Authors
- Rodius, S., de Klein, N., Jeanty, C., Sánchez-Iranzo, H., Crespo, I., Ibberson, M., Xenarios, I., Dittmar, G., Mercader, N., Niclou, S.P., Azuaje, F.
- Source
- Full text @ Sci. Rep.
|
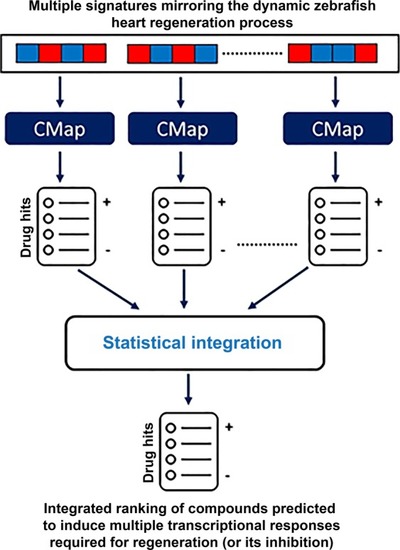
Systems-level prediction of candidate drugs with potential to induce transcriptional responses as observed |
|
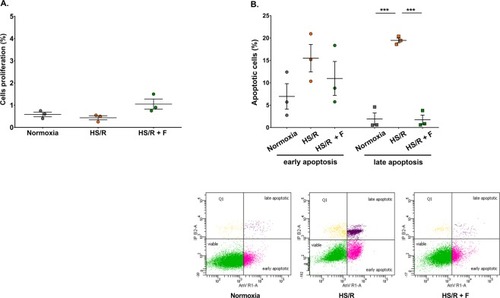
Fisetin enhanced the viability of H9c2 cardiomyocytes. ( |
|
Effect of fisetin on cardiomyocytes proliferation and apoptosis. Flow cytometry experiments were carried out to measure the amount of proliferating and apoptotic cells in each experimental group. ( |
|
Fisetin regulated a panel of genes related to cardioprotection, proliferation and maturation. Gene expression was assessed by qRT-PCR in cells cultured under normoxia, subjected to HS/R and treated with DMSO as vehicle control (HS/R) or subjected to HS/R and treated with 15 μM fisetin (HS/R + F) during 24 h. Results are expressed as the mean of four independent experiments. Statistical significance was determined using a one-way ANOVA corrected for multiple testing with a Tukey-Kramer as post-test (corrected p-value < 0.05). Statistically significant results are indicated in bold with a star. Fold change < 1: genes down-regulated compared to normoxia (yellow). Fold change > 1: genes up-regulated compared to normoxia (blue). |
|
Fisetin decreased ROS expression level in cardiomyocytes. The cell permeable, nonfluorescent dihydrorhodamine 123 probe enters the cell where it is oxidized by ROS to fluorescent rhodamine 123. Fluorescence intensity, proportional to ROS expression level, was measured by flow cytometry in each experimental group. Normoxia: control group; HS/R: cells subjected to HS/R, treated with DMSO as vehicle control; HS/R + F: cells subjected to HS/R, treated with 15 μM fisetin. Results are expressed as the mean of three independent experiments. One-way ANOVA. Post-hoc analysis by Tukey. **P ≤ 0.01. |
|
Fisetin inhibited activation of caspases 8, 9 and 3 in H9c2 cells. Caspases activity was assessed by flow cytometry using active caspase staining kits specific to caspase 8, 9 and 3, respectively. Dot plots represent the percentage of cardiomyocytes expressing activated caspase 8, 9 or 3. Normoxia: control group. HS/R: cells subjected to HS/R, treated with DMSO as vehicle control. HS/R + F: cells subjected to HS/R, treated with 15 μM fisetin. Results are expressed as the mean of three independent experiments. Two-way ANOVA. Post-hoc analysis by Tukey. **P ≤ 0.01, *P ≤ 0.05. |
|
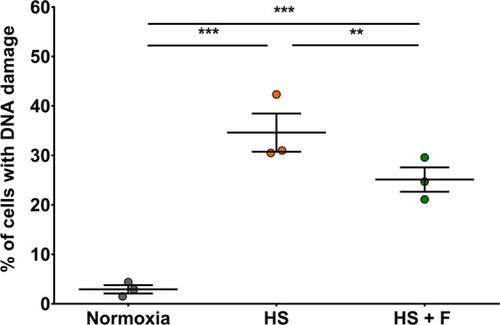
Fisetin protected cardiomyocytes cultured under HS from DNA damage. The proportion of damaged cells in each experimental group was measured by flow cytometry using the anti-8 Hydroxyguanosine antibody as DNA damage marker. Normoxia: control group. HS: cells in HS, treated with DMSO as vehicle control. HS + F: cells in HS, treated with 15 μM fisetin. Results are expressed as the mean of three independent experiments. ANOVA two factors (1: biological replicates/random; 2: Treatments). Post-hoc analysis by Tukey. ***P ≤ 0.001, **P ≤ 0.01. |
|
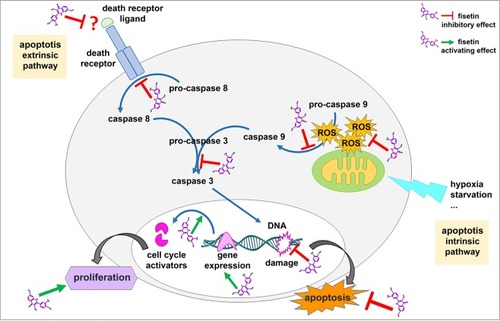
Schematic representation of fisetin cardioprotective effects on neonatal rat cardiomyocytes. Fisetin protects cells from apoptosis by targeting both intrinsic and extrinsic apoptotic pathways. Fisetin also enhances proliferation by activating expression of cell cycle activators. |