- Title
-
Autophagy Activation in Zebrafish Heart Regeneration
- Authors
- Chávez, M.N., Morales, R.A., López-Crisosto, C., Roa, J.C., Allende, M.L., Lavandero, S.
- Source
- Full text @ Sci. Rep.
|
Autophagic vesicle formation in the heart ventricle is increased during zebrafish cardiac regeneration. Electron microscopy shows a high degree of tissue remodeling of the cardiac muscle, as well as the formation of several autophagosome and phagolysosome vesicles upon apex amputation (yellow arrow heads and higher-magnification images below). An increase in the number of autophagic vesicles was above all evident in regions closer to the injured area (proximal), while also found significantly more abundant at 3 dpa distal to the amputation plane. Insets represent higher magnifications of the observed autophagic vesicles at each experimental time point. Scale bars represent 2 μm. *p ≤ 0.05. N ≥ 3. |
|
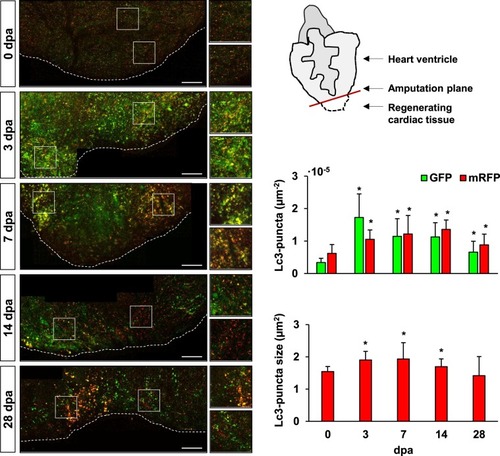
Enhanced accumulation of autophagosomes and phagolysosomes is observed in the injured cardiac tissue upon amputation. The transgenic line |
|
Western blot analysis suggests the activation of autophagy at the early stages of cardiac regeneration. The stimulation of autophagy at 3 dpa and the increased formation of autophagosomes between days 3–7 post-amputation, in comparison to sham-operated animals, is observed in the increased abundance of Beclin1, Lc3-I and Lc3-II, respectively ( |
|
Rapamycin treatment affects the inflammatory phase of the cardiac regeneration process. Zebrafish were treated with rapamycin via intraperitoneal injection two days before the amputation procedure and every second day afterwards ( |
|
Rapamycin treatment affects zebrafish cardiac regeneration. Rapamycin-treatment affected angiogenesis upon amputation, causing a negative effect in the re-vascularization rate of the regenerating zebrafish ventricle at 7 dpa ( |