- Title
-
Mechanical Forces Regulate Cardiomyocyte Myofilament Maturation via the VCL-SSH1-CFL Axis
- Authors
- Fukuda, R., Gunawan, F., Ramadass, R., Beisaw, A., Konzer, A., Mullapudi, S.T., Gentile, A., Maischein, H.M., Graumann, J., Stainier, D.Y.R.
- Source
- Full text @ Dev. Cell
|
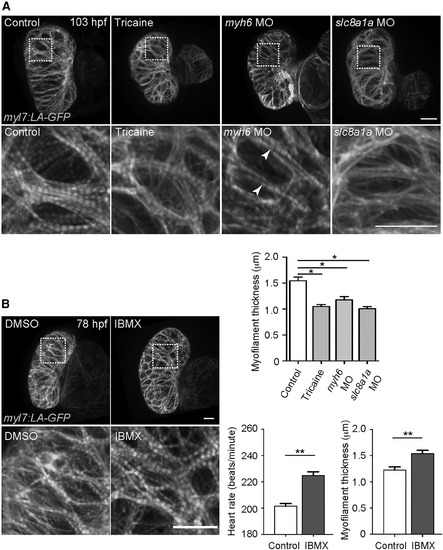
Cardiac Contractility Is Required for Myofilament Maturation (A) 3D images of 103 hpf hearts treated with or without tricaine from 72 to 103 hpf, and of 103 hpf myh6 and slc8a1a morphant hearts. myh6 morphant ventricles exhibit thinner myofilaments but a WT-like myofilament banding pattern (arrowheads). Myofilament thickness measured in 103 hpf ventricles (n = 4–5 ventricles). (B) 3D images of 78 hpf hearts treated with DMSO or IBMX from 54 to 78 hpf. 78 hpf heart rate measurement (n = 5 larvae) and ventricular myofilament thickness (n = 4–5 ventricles). Error bars, SEM. ∗p < 0.05 and ∗∗p < 0.001 by ANOVA followed by Tukey’s HSD test. Scale bars, 20 μm. |
|
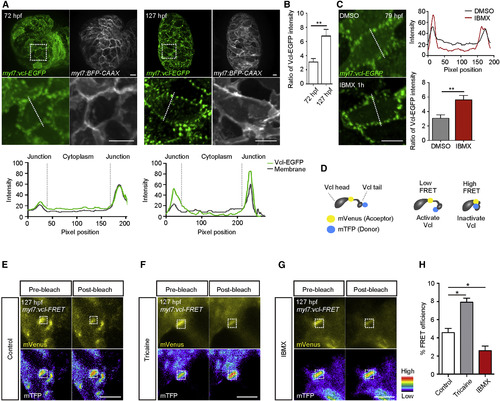
Cardiac Contractility Regulates VCL Localization and Activation (A) 3D images of 72 and 127 hpf hearts; fluorescence intensities measured along white dashed lines. (B) Ratio of 72 and 127 hpf ventricular Vcl-EGFP intensity in cell-cell junctions compared to that in the cytoplasm (n = 20 CMs). (C) 3D images of 79 hpf Tg(myl7:vcl-EGFP) ventricles treated with DMSO or IBMX; Vcl-EGFP intensity measured along white dashed lines; ratio of Vcl-EGFP intensity in cell-cell junctions compared to that in the cytoplasm (n = 20 CMs). (D) Schematic of the Vcl activation FRET biosensor (Vcl-FRET). (E–G) FRET measurement in 127 hpf Tg(myl7:vcl-FRET) ventricles immediately after cardiac arrest (E), at 15 min after starting tricaine (F) or IBMX (G) treatment; white boxes outline photobleaching region. (H) Percentage of FRET efficiencies (n = 20 ventricles per experiment). Error bars, SEM. ∗p < 0.05 and ∗∗p < 0.001 by ANOVA followed by Tukey’s HSD test. Scale bars, 10 μm in (A) and (C); 5 μm in (E)–(G). |
|
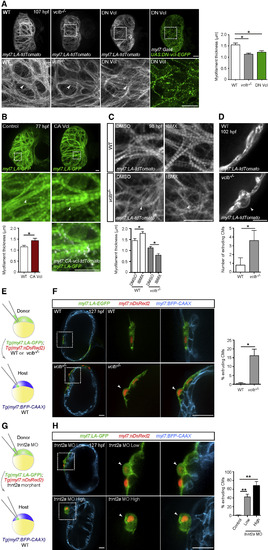
Vcl Is Required for Myofilament Maturation and Myocardial Wall Integrity in Response to Cardiac Contractility (A) 3D images of 107 hpf hearts. vclb−/− and DN Vcl-EGFP expressing CMs exhibit disorganized and thinner myofilaments than WT (arrowheads). Myofilament thickness measured in 107 hpf ventricles (n = 5 ventricles). (B) 3D images of 77 hpf hearts. CA Vcl-tdTomato+ ventricles exhibit thicker and more aligned myofilaments than WT (arrowheads). Myofilament thickness measured in 77 hpf ventricles (n = 4 ventricles). (C) 3D images of 98 hpf ventricles treated with DMSO or IBMX from 74 to 98 hpf. IBMX treatment leads to further myofilament disruption in vclb−/− ventricles than DMSO treatment (arrowheads). Myofilament thickness measured in 98 hpf ventricles (n = 4–5 ventricles). (D) Single-plane images of 102 hpf ventricles treated with IBMX from 78 to 102 hpf. IBMX treatment leads to CM extrusion (arrowhead) in vclb−/− ventricles. Number of extruding CMs measured in 102 hpf ventricles (n = 5 ventricles). (E) Schematic of the transplantation experiment. (F) Single-plane images of 127 hpf chimeric hearts treated with IBMX from 103 to 127 hpf. vclb−/− CMs extrude in chimeric hearts (arrowheads). Percentage of extruding CMs in 127 hpf chimeric ventricles (n = 5 ventricles). (G) Schematic of the transplantation experiment. (H) Single-plane images of 127 hpf chimeric hearts. tnnt2a morphant CMs extrude in chimeric ventricles (arrowheads). Percentage of extruding CMs in 127 hpf chimeric ventricles (n = 5 ventricles). Error bars, SEM. ∗p < 0.05 and ∗∗p < 0.001 by ANOVA followed by Tukey’s HSD test. Scale bars, 10 μm in (B)–(D); 20 μm in (A), (F), and (H). |
|
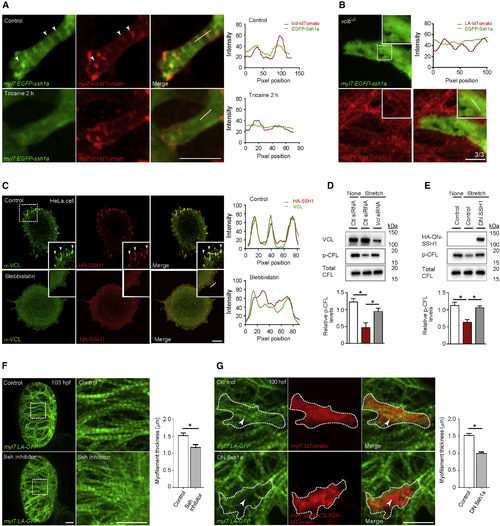
SSH1 Regulates Cardiomyocyte Myofilament Maturation (A) 3D images of a 103 hpf EGFP-Ssh1a expressing ventricle immediately after cardiac arrest and 2 h after tricaine treatment; fluorescence intensities along white dashed lines. EGFP-Ssh1a colocalizes with Vcl-tdTomato in CMs (arrowheads), but this co-localization is abrogated by tricaine treatment. (B) 3D images of a 103 hpf vclb−/− ventricle expressing EGFP-Ssh1a; fluorescence intensities along white dashed line (n = 3 animals; each animal contains 2–4 EGFP-Ssh1a+ CMs). (C) VCL and HA immunostaining of HeLa cells treated without or with blebbistatin; fluorescence intensities along white dashed lines. HA-SSH1 colocalizes with VCL (arrowheads), and blebbistatin treatment leads to loss of this co-localization. (D) Protein expression after siRNA transfection in mouse neonatal CMs exposed to cyclic stretch; relative levels of p-CFL (n = 4). (E) Protein expression in RNCMs exposed to cyclic stretch; relative levels of p-CFL (n = 3). (F) 3D images of 103 hpf hearts treated without or with Ssh inhibitor from 72 to 103 hpf. Myofilament thickness measured in 103 hpf ventricles (n = 5 ventricles). (G) 3D images of 100 hpf ventricle. DN Ssh1a overexpression leads to myofilament disruption (arrowheads). Myofilament thickness measured in 100 hpf CMs (n = 7–10 CMs). Error bars, SEM. ∗p < 0.05 by ANOVA followed by Tukey’s HSD test. Scale bars, 20 μm in (A), (B), (F), and (G); 10 μm in (C). |
|
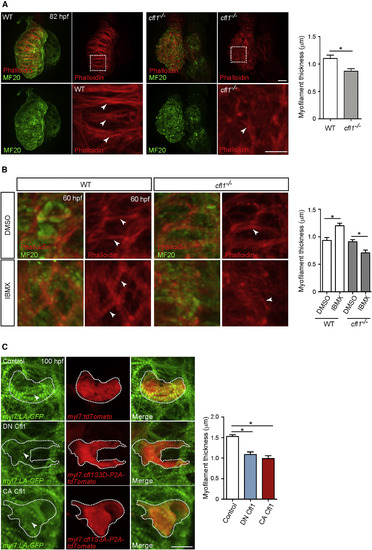
cfl1 Is Essential for Cardiomyocyte Myofilament Maturation (A) MF20 immunostaining and phalloidin staining of 82 hpf WT and cfl1−/− hearts. cfl1−/− CMs exhibit disorganized myofilaments than WT (arrowheads). Myofilament thickness measured in 82 hpf ventricles (n = 4 ventricles). (B) MF20 immunostaining and phalloidin staining of 60 hpf WT and cfl1−/− hearts treated with DMSO or IBMX from 36 to 60 hpf. IBMX treatment leads to myofilament disruption in cfl1−/− CMs than WT (arrowheads). Myofilament thickness measured in 60 hpf ventricles (n = 4 ventricles). (C) 3D images of 100 hpf ventricles. Overexpression of either DN Cfl1 or CA Cfl1 leads to myofilament disruption (arrowheads) compared to control. Myofilament thickness measured in 100 hpf ventricles (n = 6 CMs). Error bars, SEM. ∗p < 0.05 by ANOVA followed by Tukey’s HSD test. Scale bars, 20 μm. |
Reprinted from Developmental Cell, 51(1), Fukuda, R., Gunawan, F., Ramadass, R., Beisaw, A., Konzer, A., Mullapudi, S.T., Gentile, A., Maischein, H.M., Graumann, J., Stainier, D.Y.R., Mechanical Forces Regulate Cardiomyocyte Myofilament Maturation via the VCL-SSH1-CFL Axis, 62-77.e5, Copyright (2019) with permission from Elsevier. Full text @ Dev. Cell